Abstract
The full-length cDNA sequence encoding Brugia malayi L3 paramyosin has been isolated by immunoscreening a cDNA library with a mouse antiserum raised against Wuchereria bancrofti L3 infective larvae. A recombinant truncated form of paramyosin was expressed as a glutathione S-transferase fusion protein and used to evaluate humoral responses of adults from a W. bancrofti-endemic area in French Polynesia according to their parasitological status. Immunoglobulin G4 (IgG4) preferentially bound to paramyosin in W. bancrofti-parasitized individuals, in contrast to unparasitized individuals, who harbored neither microfilaria nor Og4C3 adult worm circulating antigen. Reduction of the anti-paramyosin IgG4 titer following combined chemotherapy with diethylcarbamazine and ivermectin was significantly correlated with a reduction in the adult worm burden. This indicates that the presence of paramyosin-reactive IgG4 is associated with the presence of parasites and that reduction can be used as an immunological marker for W. bancrofti clearance.
Approximately 120 million individuals are affected by lymphatic filariasis worldwide, 107 million of whom are parasitized by Wuchereria bancrofti and 13 million are parasitized by Brugia malayi (18). Epidemiological evidence suggests that exposure to infective larvae (L3) stimulates protective immunity (4). In addition, a large proportion of individuals living in areas of endemic infection (endemic individuals) appear to be free of microfilariae and adult worms, although they possess antifilarial antibodies and therefore have been exposed to parasitism (16, 17, 23). These individuals, commonly described as asymptomatic endemic normals, are presumed to be resistant to L3 infection. The characterization of antigens from the infectious stage that induce a specific response in resistant as opposed to parasitized individuals is a crucial step in understanding early resistance to filarial development and in searching for vaccine candidates (6). More directly, this should help in designing diagnostic tools for W. bancrofti and especially for B. malayi, for which sensitive tools for detecting cryptic parasitism are lacking (18). In this study, we have identified paramyosin as a major immunodominant antigen of infective larvae (L3) of B. malayi and studied the antibody responses to the recombinant paramyosin of individuals exposed to W. bancrofti transmission in terms of their parasitological status.
MATERIALS AND METHODS
Study populations.
A total of 123 patients were recruited among the adult population (>20 years old) of the island Tahaa, Society Archipelago, French Polynesia. This island has ca. 4,000 inhabitants, and a recent survey, conducted in the course of a large-scale chemotherapy assay, indicated that 22% of adult individuals had microfilariae (Mf) (13); 46% had Og4C3 circulating antigen and were thus suspected of harboring W. bancrofti adult worms (17). The patients were surveyed for a history of filariasis, given a physical examination, and screened for microfilaremia by filtration of 1 ml of venous blood. Blood was collected during the day, since W. bancrofti var. pacifica is subperiodic in French Polynesia (14). Sera were separated by centrifugation and frozen at −30°C until use. All sera were screened for the presence of adult worm circulating filarial antigen (CFA) by an Og4C3 enzyme-linked immunosorbent assay (ELISA) (12) as recommended by the manufacturer (JCU Tropical Biotechnology Pty Ltd., Brisbane, Australia). The limit for positivity was 100 U of Og4C3 antigen per ml. All endemic individuals recruited were checked for positive antifilarial serology (anti-adult Brugia malayi immunoglobulin G (IgG) (7) to determine their exposure to W. bancrofti parasitism. Nine sera from European adults who had never lived in a filariasis-endemic area and were negative for the three markers were used as the negative control (nonendemic subjects). Endemic individuals were classified into three groups: (i) Mf carriers (Mf and CFA positive), (ii) amicrofilaremic adult worm carriers (Mf negative but CFA positive), and (iii) unparasitized subjects (Mf and CFA negative). The mean age of all individuals enrolled was 48.1 years old (range, 21 to 81 years), the sex ratio was 0.48 (female/male), and the three groups were not significantly different for these two parameters.
All individuals were treated twice with a single annual dose of ivermectin at 400 μg/kg plus diethylcarbamazine at 3 mg/kg (13). Blood was collected before the first treatment (month 0), 1 year after the first treatment and just before the second treatment (month 12), and 1 year after the second treatment (month 24). Mf and CFA levels were determined at each of the three time points.
Parasite extracts and antisera.
Protein extracts were prepared from Mf or L3 larvae of W. bancrofti and from B. malayi adult worms as follows. W. bancrofti L3 larvae were obtained from Aedes polynesiensis mosquitoes previously fed on human blood collected from a microfilaremic individual. Mf were purified from human blood on a Percoll gradient (Sigma Co., St. Louis, Mo.). B. malayi adult worms were provided by E. A. Ottesen (National Institutes of Health, Bethesda, Md.). All parasites were kept in phosphate-buffered saline (PBS) and stored at −30°C before use. Crude protein extracts were made by sonicating the parasites in PBS, and the protein concentration was determined after centrifugation by the bicinchoninic acid protein assay (Pierce, Rockford, Ill.). Mouse polyclonal serum was raised against each filarial stage in BALB/c mice by one intraperitoneal injection of 50 μg of protein extract in complete Freund’s adjuvant, followed by three intraperitoneal injections of 50 μg of protein in incomplete Freund adjuvant at 2- to 3-week intervals. The mice were bled 1 week after the last injection.
Isolation of paramyosin cDNA.
A B. malayi L3 cDNA library constructed in the lambda UniZap XR vector (Stratagene) was provided by S. A. Williams, Smith College, Northampton, Mass. (SAW94WL-BmL3 library) (2) and screened with the antiserum raised against W. bancrofti L3. Immunoscreening was performed with horseradish peroxidase-labelled goat anti-mouse IgG heavy plus light chain (Biosys) as the secondary antibody and developed with α-chloronaphthol. The positive clones were purified by repeated cycles of immunoscreening, and the size of the B. malayi cDNA inserts was determined by PCR with T3 and T7 pBluescript primers. Recombinant pBluescript plasmids were excised from positive phages, and partial nucleotide sequencing of the 5′ and 3′ ends of the inserts was performed with the Circumvent thermal cycle DNA sequencing kit (New England Biolabs, Beverly, Mass.).
Sequence analysis.
Two inserts of 3 kb (BmILM5) and 2.1 kb (BmILM6), which showed high homology to paramyosins from other filarial species, were selected, and their nucleotide sequence was determined with the Circumvent thermal cycle DNA sequencing kit and ExoVent polymerase (New England Biolabs). Sequencing of full-length cDNA in both directions was done with synthetic primers (Genosys, Cambridge, United Kingdom) designed from the paramyosin nucleotide sequences of Onchocerca volvulus and B. malayi cDNAs. Initial assembly of the cDNA sequences and amino acid translations were performed with the sequence analysis software PC-Gene (IntelliGenetics Inc., Mountain View, Calif.). The nucleotide and protein sequences were compared with all other sequences in database searches with the BLASTN and the BLASTX search programs (1). One of the cDNAs (BmILM5) encoded the full-length paramyosin of B. malayi.
Expression and purification of recombinant paramyosins.
The coding sequences of BmILM5 and BmILM6 cDNAs were amplified by PCR with the forward primer 5′-GCGGATCCGATGTCCGGTTCAC-3′ and the reverse primer 5′-CGCGAAGCTTGGGTACCGGGCCC-3′. The PCR products were subcloned between the BamHI and HindIII sites of the expression plasmid vector pGEX-FA (derived from pGEX-3T, a gift from F. Laurent, Nouzilly, France), downstream of the glutathione S-transferase (GST) coding sequence. Sequences at the sites of ligations were checked by sequencing with 3′-pGEXRev and 5′-pGEXFor primers (Pharmacia, Uppsala, Sweden). Recombinant GST fusion proteins were expressed in Escherichia coli DH5α after induction with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Expression of recombinant fusion proteins was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (11% polyacrylamide gels) of lysed bacterial cell pellets. Recombinant proteins were visualized by Coomassie blue staining and by using anti-GST polyclonal rabbit antibody after transfer onto nitrocellulose membranes (Hybond-ECL; Amersham, Arlington Heights, Ill.). Bacterial cultures were sonicated, and GST fusion proteins (and control GST) were purified by affinity chromatography with glutathione agarose by the protocols described by the manufacturer (Pharmacia). The homogeneity of purified proteins was checked by SDS-PAGE and Coomassie blue staining, and the protein concentration was determined by the bicinchoninic acid protein assay (Pierce).
ELISAs with recombinant paramyosin and human sera.
The sera from endemic and nonendemic individuals were screened for total IgG or IgG isotype reactivity to recombinant BmILM6 truncated paramyosin as follows. Microtiter plates (Luxlon@ M29 LES; CEB) were coated in parallel with purified GST-BmILM6 fusion protein or purified GST at 0.1 μg/well for IgG assays or 0.5 μg/well for IgG subclass assays. The plates were incubated for 2 h at 37°C, stored overnight at 4°C, and blocked before use in ELISA by a 2-h incubation in 0.05% Tween 20–10% goat serum in PBS. After the plates were washed with 0.05% Tween 20 in PBS, patient sera diluted 1/100 in the same diluent (ELISA diluent) were added in duplicate, and the plates were incubated for 2 h at 37°C. The plates were washed before the addition of murine monoclonal anti-human IgG Fc (clone HP 6017; Sigma) diluted 1/1,000. After a 2-h incubation at room temperature (ca. 25°C), all the wells were washed and received a 1/2,000 horseradish peroxidase-conjugated rabbit anti-mouse reagent (Biosys); the plates were then incubated for 2 h at room temperature. After the plates were washed, o-phenylenediamine substrate (OPDA; Sigma) was added to each well for 30 min and the optical density (OD) was recorded at 492 nm. Preliminary assays to determine the appropriate dilutions of patient serum, antibodies, horseradish peroxidase and conjugates were carried out with a pool of 15 sera from microfilaremic Polynesian individuals used as positive controls and a pool of 5 sera from nonendemic European individuals as negative controls. The ELISA for IgG isotype analysis was carried out similarly, with the following isotype-specific mouse monoclonal antibodies (Sigma): IgG1, clone HP-6001; IgG2, clone HP-6002; IgG3, clone HP-6047; and IgG4, clone HP-6023 (all used at a dilution 1/2,000).
Positive and negative sera were included on all plates and used to adjust for minor plate-to-plate variations. The arithmetic mean of the OD from duplicate wells coated with GST were subtracted from the OD means from wells coated with GST-BmILM6 to give a net OD. The cutoff levels were defined as the arithmetic means ± 2 SD of the antibody activities of sera from nonendemic individuals. Anti-paramyosin IgG4 titers of positive sera were measured at a range of four or more dilutions by comparison to a standard curve obtained with the same pool of 15 microfilaremic individuals mentioned above.
Statistical analysis.
Differences in net OD means between patient groups were analyzed by the Student t test or analysis of variance. Correlation between covariates was analyzed by Spearman’s correlation test. Statistical significance by any of these methods was inferred as P < 0.05.
Nucleotide sequence accession number.
The sequence of the BmILM5 cDNA was submitted to GenBank under accession no. U77590.
RESULTS
Analysis of full-length paramyosin cDNA.
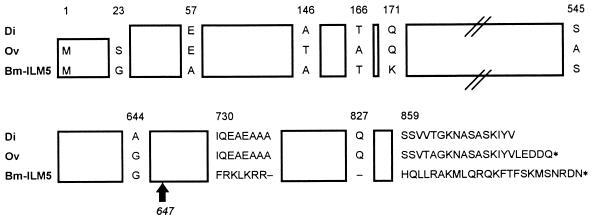
About 100,000 recombinant phages were screened with the anti-B. malayi L3 mouse polyclonal antiserum. Twelve strongly positive clones were purified for further analysis. The insert sizes, determined by PCR with T3 and T7 primers, were mostly of 3 kb, except for one insert, which was 2.1 kb. Partial sequencing and restriction enzyme analysis indicated that all recombinant plasmids had similar 5′-end nucleotide sequences and all had strong homologies to the paramyosin of O. volvulus. Therefore, two clones with inserts of 2.1 and 3 kb (referred to as clones BmILM6 and BmILM5) were selected for complete nucleotide sequencing. Clone BmILM5 contained the full-length cDNA of B. malayi paramyosin and consisted of an open reading frame of 2,640 nucleotides predicted to encode 880 amino acids containing a 97-kDa protein. Alignments of BmILM5 and all other sequences in GenBank showed 96% protein homology to the paramyosin of two other filarial parasites, O. volvulus (accession no. M95813) and Dirofilaria immitis (J04009). This is schematically represented in Fig. 1. BmILM5 differed from the paramyosin sequences of the other species at the COOH-terminal end, with two divergent blocks in the last 150 amino acids. The nucleotide sequence of BmILM6 was identical to that of BmILM5 but lacked 699 nucleotides at the 3′ end and therefore encoded a protein of only 647 amino acids.
FIG. 1.
Schematic alignment of the predicted amino acid sequence comparison of full-length BmILM5 and the sequences of paramyosin from D. immitis (Di) and O. volvulus (Ov). The sequence of BmILM5 is one continuous open reading frame encoding 880 amino acids, while O. volvulus paramyosin consists of 879 residues and the D. immitis sequence is missing the NH2 and COOH extremities. Regions containing amino acids identical to the published sequences of O. volvulus and D. immitis paramyosins are boxed. Stop codons are indicated by asterisks. Missing residues are shown by dashes. The arrow indicates the COOH end of BmILM6.
Expression of GST fusion paramyosin.
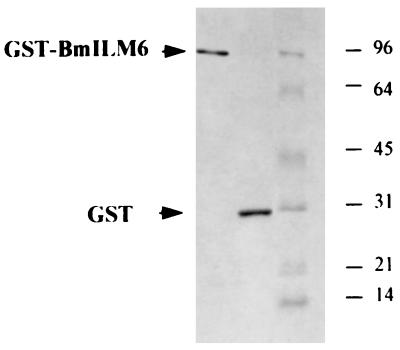
The cDNAs of BmILM5 and BmILM6 were subcloned into the pGEX-FA expression vector to produce GST fusion proteins. E. coli cells transformed with pGEX-FA/BmILM5 produced a single fusion protein with a molecular mass of about 97 kDa when reacted with W. bancrofti L3 antiserum and with other filarial-stage antisera and anti-GST antiserum (data not shown). The GST-BmILM5 protein is smaller than the predicted size of the fusion protein (126 kDa), indicating that the protein was not fully expressed or that the product was unstable. In contrast, E. coli cells transformed with pGEX-FA/BmILM6 produced a major 100-kDa fusion protein, as expected, and two minor bands at 76 and 60 kDa, recognized by anti-GST antibodies (data not shown), suggesting that they are proteolysis products of the 100-kDa fusion protein. The three proteins were also recognized by antibodies raised against W. bancrofti L3, W. bancrofti Mf, or B. malayi adult worms (data not shown). The recombinant GST expressed in bacterial cells transformed with pGEX-FA alone was not recognized by antifilarial antisera. No reactivity was observed with nonimmune mouse serum, showing that recombinant paramyosin was specifically recognized by filaria-infected hosts. The 100-kDa GST-BmILM6 fusion protein and the GST were purified with glutathione-agarose. Their homogeneity were checked by SDS-PAGE and Coomassie blue staining (Fig. 2).
FIG. 2.
SDS-PAGE of the GST-BmILM6 fusion protein and recombinant GST expressed in E. coli and purified on glutathione-agarose. Proteins (20 μg per lane) were stained with Coomassie blue. The positions of the molecular mass markers (in kilodaltons) are indicated on the right.
Antibody responses of W. bancrofti endemic individuals to GST-BmILM6.
Since expression of the full-length cDNA fragment (BmILM5) was inadequate in pGEX-FA, we used recombinant truncated paramyosin (GST-BmILM6) to analyze the humoral response of endemic individuals to paramyosin.
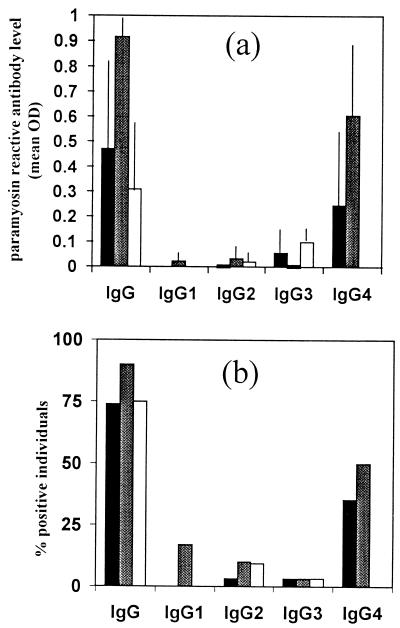
None of the nonendemic individuals had isotype antibody responses to BmILM6. The reactivity of the sera from endemic individuals to paramyosin is illustrated in Fig. 3. All endemic individuals recruited in this study had been exposed to W. bancrofti parasites since all had anti-filarial IgG, as evaluated by ELISA with B. malayi adult worm extract (data not shown). The percentage of endemic individuals positive for antiparamyosin IgG ranged from 75 to 90%, but the level of IgG was significantly higher in individuals who had only W. bancrofti adult worms than in individuals with Mf or unparasitized individuals (P < 0.05). IgG1, IgG2, and IgG3 antibody responses of the three groups to BmILM6 were low (mean OD, < 0.1), and the proportion of positive individuals ranged from 0 to 17%. In contrast, the IgG4 antibody response showed an interesting pattern. The presence of paramyosin-reactive IgG4 was observed only in parasitized individuals (35% of Mf-positive individuals and 50% of Mf-negative, CFA-positive individuals), with a significantly higher response in Mf-negative, CFA-positive individuals (P < 0.01). The level of CFA in Mf-positive individuals was four times higher than that in Mf-negative individuals. There was a negative correlation between the CFA level in parasitized individuals (Mf positive and negative, n = 26) and the paramyosin-reactive IgG4 level (r = −0.55). However, no correlation was observed between paramyosin-reactive IgG4 level and Mf status. Neither the CFA level nor the paramyosin-reactive IgG4 level was correlated with the age of the individuals.
FIG. 3.
Ig responses of endemic individuals to recombinant paramyosin (BmILM6) according to their W. bancrofti parasitological status: Mf positive, CFA positive (solid histograms, n = 32); Mf negative, CFA positive (shaded histograms, n = 30); Mf negative, CFA negative (open histograms, n = 31). (a) BmILM6 IgG and isotype antibody levels (expressed as mean OD492 values). Vertical lines represent 95% confidence intervals. (b) Percentage of individuals positive in anti-BmILM6 IgG and isotypes.
Decline of anti-paramyosin antibody levels after chemotherapy.
The decrease in paramyosin-reactive IgG4 titers was monitored after each of two annual treatments with diethylcarbamazine (3 mg/kg) and ivermectin (400 μg/kg) in parasitized individuals who were anti-paramyosin IgG4 positive before treatment (n = 26). There was a sharp and continuous reduction in anti-paramyosin IgG4 titers, which was highly correlated (r = 0.99) with the reduction in the adult worm burden (Table 1).
TABLE 1.
Evolution of titer and prevalence of CFA and paramyosin-reactive IgG4 in W. bancrofti-parasitized individuals before and after filaricidal drug treatments
| Time (mo) after treatment | Meana CFA level (range) | % Residual antigen levelb | Meana paramyosinreactive IgG4 titer (range) | % Residual paramyosin-reactive IgG4b |
|---|---|---|---|---|
| 0c | 1,874 (100–8,778) | 100 | 704 (26–7,742) | 100 |
| 12 | 713 (0–8,124) | 51 | 221 (5–2,267) | 37 |
| 24 | 479 (0–7,816) | 35 | 111 (5–1,763) | 24 |
Geometric mean.
Arithmetic mean of individual residual antigen levels or IgG4 titers.
Before the first treatment (see Materials and Methods).
DISCUSSION
Immunoscreening a cDNA library from infective larvae of B. malayi has confirmed that paramyosin is a major immunodominant antigen in infective larvae. We have cloned and sequenced the full-length cDNA encoding B. malayi paramyosin. This protein shows 96% amino acid identity to the paramyosin of two other filarial parasites, D. immitis, the dog heartworm (5), and O. volvulus, the etiological agent of river blindness in humans (11). The D. immitis and O. volvulus proteins have 99% amino acid identity. The less conserved part of the protein is the carboxy-terminal end. The sequence of the cDNA isolated here confirms the partial cDNA sequence of B. malayi paramyosin already published by Li et al. (9), which was restricted to amino acids 251 to 659. The paramyosin cDNA from B. malayi showed only 33% homology to paramyosin cDNAs of the trematodes Schistosoma mansoni and S. japonicum (19, 24).
Paramyosin has been characterized as a myofibrillar protein found only in invertebrates. In B. malayi, it is located within the longitudinal somatic musculature of L3, L4, and adult worms (21). Here we show that it is expressed in Mf as well. In an attempt to search for protective antigens in filarial infections, Nanduri and Kazura (15) observed that paramyosin immunization of mice could induce the clearance of B. malayi Mf. Moreover, B. malayi recombinant truncated paramyosin conferred partial protection against infection in immunized jirds (10). Therefore, paramyosin is considered a candidate component for a vaccine to prevent the development of filariasis. Paramyosin is also a strong candidate for a vaccine for S. mansoni infections, where both native and recombinant forms of paramyosin have been shown to be immunogenic and to confer protective immunity in mice (8, 19, 20). In addition, nucleic acid vaccination with the paramyosin coding sequence elicited the production of anti-paramyosin antibodies in mice (25).
Attempts to express the entire coding region in the pGEX-FA expression vector were unsuccessful, because of either premature termination or proteolysis in E. coli. However, the truncated form of recombinant paramyosin (the amino-terminal end) reacted strongly with sera directed against each stage of the filarial parasites W. bancrofti and B. malayi. The cross-reactivity between B. malayi paramyosin and antisera raised against W. bancrofti can be explained by the high homology between B. malayi and W. bancrofti paramyosin sequences (23a). Steel et al. (22) have reported that the main domain of O. volvulus paramyosin reactivity is located at the first 120 amino acids of the molecule. This is consistent with our results, since BmILM6 lacked 699 nucleotides (233 amino acids) at its 3′ end, the region shown to be divergent in filarial species (7).
Although the sequence of paramyosin from B. malayi was highly homologous to that of paramyosin from O. volvulus, the pattern of humoral responses of Polynesian individuals was different from that observed with O. volvulus-endemic individuals from Guatemala or Ghana (22). Individuals living in areas of endemic onchocerciasis infection had a strong IgG3 response but a very weak IgG4 response. In our study, lymphatic filariasis-endemic individuals had a strong IgG4 response while other isotypes were poorly reactive. The most interesting data from our study was the specific IgG4 response in individuals parasitized with W. bancrofti (Mf and/or adult worms) and the lack of response in parasite-free individuals (commonly referred to as putatively immune individuals). By contrast, Polynesian individuals who are free of W. bancrofti parasitism (Mf and Og4C3 antigen negative), although subjected to transmission, can harbor IgG4 reacting with crude filarial extracts (17a). Therefore, the specificity of the anti-paramyosin IgG4 response with parasitism and the correlation of anti-paramyosin IgG4 titers and reduction of adult worm antigen following chemotherapy make paramyosin-reactive IgG4 detection a good marker of parasitism and efficacy of control. Due to the high homology of the B. malayi and W. bancrofti antigens, it is likely that the recombinant antigen identified in our work will be useful for diagnosis of B. malayi as well. In particular, it may be useful to detect cryptic parasitism due to adult worms. Other recombinant B. malayi antigens that raise an IgG4 response have also been identified as markers of parasitism in brugian and bancroftian filariasis (3), and further studies must be carried out in this direction to design a cocktail of recombinant antigens sensitive enough for a B. malayi parasitism survey, as has been done for O. volvulus.
ACKNOWLEDGMENTS
This work was supported by the Institut Malardé.
We thank L. Moilon for help in immunization of mice; F. Laurent and M. Guillotte-Blisnick for providing pGexFA plasmid and anti-GST antiserum, respectively; and L. N. Nguyen and J. P. Moulia-Pelat for human blood samples. Thanks are due to E. Chungue and B. Murgue for helpful discussion, to G. Milon and O. Puijalon for helpful discussion and for criticizing the manuscript, and to anonymous reviewers for improving the manuscript.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Blaxter M L, Raghavan N, Ghish I, Guiliano D, Lu W, Williams S A, Slatko B, Scott A L. Genes expressed in Brugia malayi infective third stage larvae. Mol Biochem Parasitol. 1996;77:77–93. doi: 10.1016/0166-6851(96)02571-6. [DOI] [PubMed] [Google Scholar]
- 3.Chandrashekar R, Curtis K C, Ramzy R M, Liftis F, Li B W, Weil G J. Molecular cloning of Brugia malayi antigens for diagnosis of lymphatic filariasis. Mol Biochem Parasitol. 1994;64:261–271. doi: 10.1016/0166-6851(94)00035-2. [DOI] [PubMed] [Google Scholar]
- 4.Day K P, Gregory W F, Maizels R M. Age specific acquisition of immunity to infective larvae in a bancroftian filariasis endemic area of Papua New Guinea. Parasite Immunol. 1990;13:227–290. doi: 10.1111/j.1365-3024.1991.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 5.Grandea A G, Tuyen K, Asikin N, Davis T B, Philipp M, Cohen C. A lambda gt11 cDNA recombinant that encodes Dirofilaria immitis paramyosin. Mol Biochem Parasitol. 1989;35:31–42. doi: 10.1016/0166-6851(89)90139-4. [DOI] [PubMed] [Google Scholar]
- 6.Grieve R B, Wisnewski N, Frank G R, Tripp C A. Vaccine research and development for the prevention of filarial nematode infections. In: Powell M F, Newman M J, editors. Vaccine design: the subunit and adjuvant approach. New York, N.Y: Plenum Press; 1995. pp. 737–768. [DOI] [PubMed] [Google Scholar]
- 7.Lal R B, Ottesen E A. Enhanced diagnostic specificity in human filariasis by IgG4 antibody assessment. J Infect Dis. 1988;158:1034–1037. doi: 10.1093/infdis/158.5.1034. [DOI] [PubMed] [Google Scholar]
- 8.Lanar D E, Pearce E J, James S L, Sher A. Identification of paramyosin as schistosome antigen recognized by intradermally vaccinated mice. Science. 1986;234:593–596. doi: 10.1126/science.3094144. [DOI] [PubMed] [Google Scholar]
- 9.Li B W, Chandrashekar R, Alvarez R M, Liftis F, Weil G J. Identification of paramyosin as a potential protective antigen against Brugia malayi infection in jirds. Mol Biochem Parasitol. 1991;49:315–324. doi: 10.1016/0166-6851(91)90075-h. [DOI] [PubMed] [Google Scholar]
- 10.Li B W, Chandrashekar R, Weil G J. Vaccination with recombinant filarial paramyosin induces partial immunity to Brugia malayi infection in jirds. J Immunol. 1993;150:1881–1885. [PubMed] [Google Scholar]
- 11.Limberger R J, McReynolds L A. Filarial paramyosin: cDNA sequences from Dirofilaria immitis and Onchocerca volvulus. Mol Biochem Parasitol. 1990;38:271–280. doi: 10.1016/0166-6851(90)90030-p. [DOI] [PubMed] [Google Scholar]
- 12.More S J, Copeman D B. A highly specific and sensitive monoclonal antibody based ELISA for the detection of circulating antigen in bancroftian filariasis. Trop Med Parasitol. 1990;41:403–406. [PubMed] [Google Scholar]
- 13.Moulia-Pelat J P, Glaziou P, Weil G J, Nguyen L N, Gaxotte P, Nicolas L. Combination ivermectin plus diethylcarbamazine, a new tool for control of lymphatic filariasis. Trop Med Parasitol. 1995;46:9–12. [PubMed] [Google Scholar]
- 14.Moulia-Pelat J P, Glaziou P, Chanteau S, Nguyen L N, Marcet Y, Cardines R, Martin P M V, Cartel J L. Periodicity of Wuchereria bancrofti var. pacifica filariasis in French Polynesia. Trop Med Parasitol. 1993;44:83–85. [PubMed] [Google Scholar]
- 15.Nanduri J, Kazura J W. Paramyosin-enhanced clearance of Brugia malayi microfilaremia in mice. J Immunol. 1989;14:3359–3363. [PubMed] [Google Scholar]
- 16.Nicolas L. New tools for diagnosis and monitoring of Wuchereria bancrofti parasitism: the Polynesian experience. Parasitol Today. 1997;13:370–375. doi: 10.1016/s0169-4758(97)01125-3. [DOI] [PubMed] [Google Scholar]
- 17.Nicolas L, Plichart C, Nguyen N L, Moulia-Pelat J P. Reduction of Wuchereria bancrofti adult worm circulating antigen after annual treatments of diethylcarbamazine and ivermectin on in French Polynesia. J Infect Dis. 1997;171:489–492. doi: 10.1093/infdis/175.2.489. [DOI] [PubMed] [Google Scholar]
- 17a.Nicolas, L., et al. Unpublished data.
- 18.Ottesen E A, Ramachandran C P. Lymphatic filariasis infection and disease: control strategies. Parasitol Today. 1995;11:129–131. [Google Scholar]
- 19.Pearce E J, James S L, Dalton J, Barrall A, Ramos C, Strand M, Sher A. Immunochemical characterization and purification of Sm-97, a Schistosoma mansoni antigen monospecifically recognized by antibodies from mice protectively immunized with a nonliving vaccine. J Immunol. 1986;137:3593–3600. [PubMed] [Google Scholar]
- 20.Ramirez B L, Kurtis J D, Wiest P M, Arias P, Aligui F, Acosta L, Peters P, Olds G R. Paramyosin: a candidate vaccine antigen against Schistosoma japonicum. Parasite Immunol. 1996;18:49–52. doi: 10.1046/j.1365-3024.1996.d01-4.x. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz K A, Hale T J, Rajan T V, Yates J A. Localization of paramyosin, myosin, and a heat shock protein 70 in larval and adult Brugia malayi. J Parasitol. 1996;82:367–370. [PubMed] [Google Scholar]
- 22.Steel C, Limberger R J, McReynolds L A, Ottesen E A, Nutman T B. B cell responses to paramyosin. Isotypic analysis and epitope mapping of filarial paramyosin in patients with onchocerciasis. J Immunol. 1990;145:3917–3923. [PubMed] [Google Scholar]
- 23.Weil G J, Jain D C, Santhanam S, Malhotra A, Kumar H, Sethumadhavan K V P, Liftis F, Ghosh T K. A monoclonal antibody-based enzyme immunoassay for detecting parasite antigens in Bancroftian filariasis. J Infect Dis. 1987;156:350–355. doi: 10.1093/infdis/156.2.350. [DOI] [PubMed] [Google Scholar]
- 23a.Williams, S. A. Unpublished data.
- 24.Yang W, Waine G J, Sculley D G, Liu X, McManus D P. Cloning and partial nucleotide sequence of Schistosoma japonicum paramyosin: a potential vaccine candidate against schitosomiasis. Int J Parasitol. 1992;22:1187–1191. doi: 10.1016/0020-7519(92)90041-i. [DOI] [PubMed] [Google Scholar]
- 25.Yang W, Waine G J, McManus D P. Antibodies to Schistosoma japonicum (Asian bloodfluke) paramyosin induced by nucleic acid vaccination. Biochem Biophys Res Commun. 1995;212:1029–1039. doi: 10.1006/bbrc.1995.2073. [DOI] [PubMed] [Google Scholar]