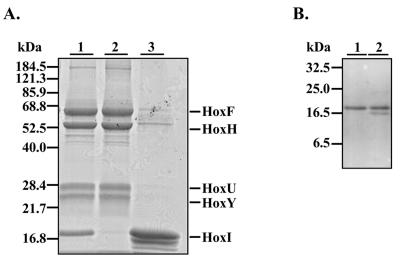
FIG. 2.
Purification of the SH and release of HoxI. (A) The Coomassie blue-stained SDS-12.5% PAGE gel shows the SH subunits HoxF, HoxH, HoxU, and HoxY and the accessory protein HoxI. Purified proteins (5 μg) were applied to each lane. Enzyme after anionic-exchange chromatography and gel filtration in 50 mM KPi buffer (pH 7) is shown in lane 1. After purification, the enzyme was subjected to additional gel filtration in 50 mM Tris-HCl (pH 8.0). Two protein peaks were observed: the high-molecular-mass peak contained tetrameric SH (lane 2), and the low-molecular-mass peak contained the accessory protein HoxI (lane 3). (B) The SH-HoxI complex after gel filtration at pH 7 (see panel A, lane 1) and the HoxI fraction after dissociation of the complex during additional gel filtration in 50 mM Tris-HCl buffer at pH 8 (Fig. 2A, lane 3) were subjected to immunoblot analysis using the Anti-HoxI antibody. Purified proteins (5 μg) were applied to each lane. Lane 1, SH-HoxI complex; lane 2, purified HoxI.