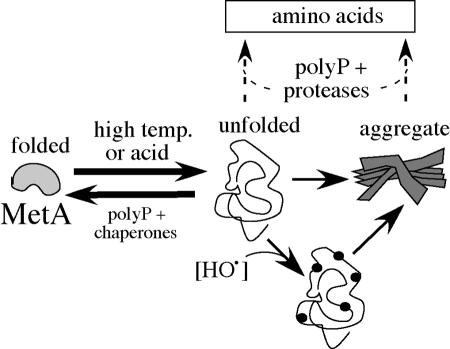
FIG. 9.
A model for the role of polyP in resistance of MetA to acid or heat. MetA exists in cells in an equilibrium between a native and a reversibly unfolded conformation (48). The unfolded protein can also form an insoluble aggregate (23). The more surface-exposed unfolded form is proposed to be susceptible to oxidation (16) and to proteolysis or aggregation. The unfolded form is favored at high temperature or low pH. Chaperones may favor refolding. PolyP is proposed to favor refolding and/or proteolysis of denatured protein or its aggregates.