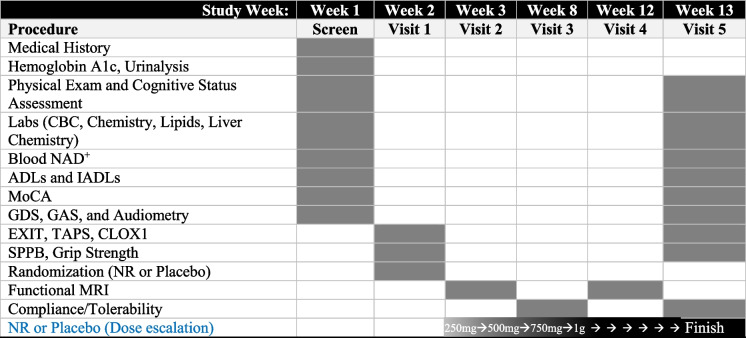
Fig. 1.
Study design and timeline. The primary objective of the study was to assess safety and tolerability of NR in older adults with MCI. The primary outcome was change in cognition as measured by MoCA. CBC, complete blood count; ADLs, activities of daily living; IADLs, instrumental activities of daily living; MoCA, Montreal Cognitive Assessment; GDS, Geriatric Depression Scale; GAS, Geriatric Anxiety Scale; EXIT, executive interview; TAPS, test of auditory processing skills; CLOX, executive clock drawing task; MRI, magnetic resonance imaging; NR, nicotinamide riboside. Shaded cells indicate procedures were conducted during the study visit. Dose escalation strategy: 250 mg × 1 week, 500 mg × 1 week, 750 mg × 1 week, and 1 g to end of study (6 weeks)