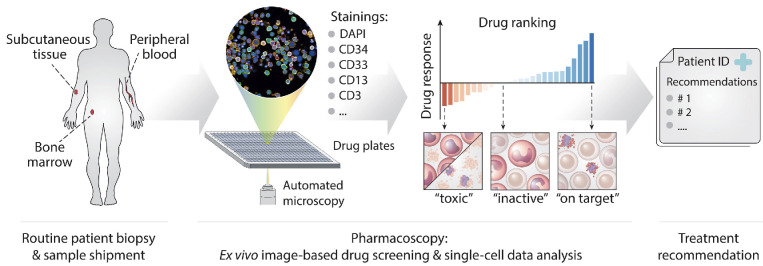
Figure 1.
Pharmacoscopy workflow for acute myeloid leukemia at relapse. Patient samples (bone marrow draws, peripheral blood, or subcutaneous/skin samples) were shipped by courier to the pharmacoscopy laboratory. There, the cells from the samples were processed by either density centrifugation (blood and bone marrow) or tissue dissociation (skin) and seeded into 384-well imaging plates, with each well containing a chemo- or immunotherapeutic compound from our test library. The plates were then incubated overnight. Immunofluorescence stainings against specific surface antigen characteristics of the patient’s leukemic cells were used to distinguish between healthy cells and malignant blasts. The cells were then subjected to automated confocal microscopy (Opera Phenix, Perkin Elmer) and image analysis using nuclear morphology to quantify the viability of malignant and healthy cells, respectively. Based on this readout, the ex vivo blast reduction capacity (peritoneal cancer index [PCY] score) was calculated for each compound. Pharmacoscopy reports were provided to the treating oncologists in the form of a short list of top-scoring drugs ranked by their predicted efficacy, as well as complete drug response profiles. The selection of treatment regimens was subsequently based on the pharmacoscopy report and the availability of compounds listed therein. If none of the listed compounds could be made available within a reasonable time frame, therapy regimens were chosen based on previously established in-house guidelines at our department.