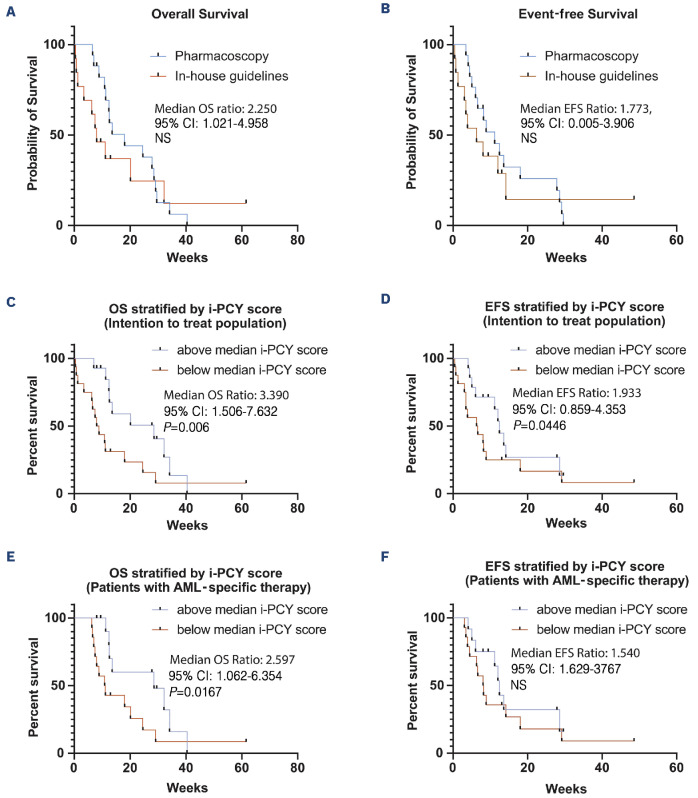
Figure 2.
Patients outcome. (A) Kaplan-Meier estimates of overall survival (OS) in the intention-to-treat population stratified by whether patients received a pharmacoscopy-derived treatment regimen (17 screening instances) or a regimen based on in-house guidelines (13 screening instances). (B) Kaplan-Meier estimates of event-free survival (EFS) in the intention-to-treat population stratified by whether patients received a pharmacoscopy-derived treatment regimen or not (17 vs. 13 screening instances). (C) Kaplan-Meier estimates of OS in the intention-to-treat population stratified by the ex vivo blast reduction capacity of their respective treatment regimen (integrated peritoneal cancer index [i-PCY] score) (14 screening instances with above median i-PCY score and 16 below). (D) Kaplan-Meier estimates of EFS in the intention-to-treat population stratified by the i-PCY score of their respective treatment regimen (14 vs. 16 screening instances). (E) Kaplan-Meier estimates of OS in patients receiving an acute myeloid leukemia (AML)-specific therapy (excluding patients receiving best supportive care) stratified by the i-PCY score of their respective treatment regimen (12 screening instances with above median i-PCY score and 14 below). (F) Kaplan-Meier estimates of EFS in patients receiving an AML-specific therapy stratified by the i-PCY score of their respective treatment regimen (12 vs. 14 screening instances). All P values were calculated by Gehan-Breslow-Wilcoxon test. CI: confidence interval; NS: not significant.