Abstract
Urinalysis was performed on 41 cats with no history of urinary tract disease. Samples were divided into aliquots, stored under differing condition and then examined for the presence of crystalluria. Crystalluria was detected in at least one stored sample in 92% of cats fed a mixed wet/dry food diet compared to 24% in the fresh sample. Crystalluria was not detected in any sample or aliquot from cats fed all wet food diets.
Urinalysis is a diagnostic test frequently used to establish a minimum database in sick cats particularly those with signs suggestive of renal or urinary tract disease. Struvite crystalluria is commonly observed but its interpretation is difficult even when associated with signs of cystitis. Many authors have questioned the significance of a diagnosis of crystalluria ex vivo (Osborne et al 1996b). Of 470 cats seen during the period of survey at the University of Bristol teaching hospital, 78 (17%) had urinalysis performed. Of these 26 (33%) had crystalluria reported with struvite being found in the majority (85%) of positive samples. Only eight (2%) of these cats were referred for lower urinary tract problems.
Due to the relatively low rate of achieving a specific diagnosis (less than 50% of cases [Ling et al 1990, Osborne et al 1996a]) in cats presenting with urinary tract signs (dysuria, haematuria), the presence of struvite crystalluria in the absence of a clear cause of cystitis has prompted treatment by dietary management. Nutritional management involving acidifying, low magnesium, low phosphorus diets is used on the basis that crystalluria may be contributing to the clinical signs and that such dietary therapy is benign. However, the long term use of poorly formulated, acidifying diets can be associated with health risks. These include potassium depletion (which can lead to renal failure) and hypercalciuria (which can lead to calcium containing urolith formation) (DiBartola et al 1993). The risk of calcium oxalate urolithiasis developing is further increased by the urinary conditions created by many such diets and their widespread use may, in part, explain the increasing incidence of calcium oxalate urolithiasis that has been reported (Osborne et al 1994). Dietary therapy is potentially of great value in the treatment of struvite urolithiasis but its indiscriminate use in cats presenting with crystalluria, without evidence of urolithiasis, is difficult to justify particularly if their presence does not reflect the situation in vivo. A study was designed to evaluate the reliability of the diagnosis of struvite crystalluria in stored urine samples.
Materials and methods
As part of a programme of health evaluations, morning urine samples were obtained by cystocentesis from 41, entire, specific pathogen free (39 domestic short hair, 2 Abyssinian cross; 12 male, 29 female) cats that had not been fed since the previous day. None of the cats had had a history of urinary tract disease. The sample was maintained at body temperature and submitted for routine urinalysis within 15 min of collection and constituted the fresh sample. The remainder was divided into 2 ml aliquots and subjected to varying storage conditions (Table 1).
Table 1.
Aliquot storage conditions
| Aliquot | Storage method | Description |
|---|---|---|
| 1 | Fresh | Baseline sample—analysed at time 0 |
| 2 | Bench | Analysed after being left in the light at room temperature for four hours |
| 3 | Fridge | Analysed immediately after being refrigerated for four hours |
| 4 | Bench & fridge | Analysed after being left in the light at room temperature for one hour and then refrigerated for four hours |
| 5 | Postal | Analysed after handling to mimic first class postage—parcelled, left in a vehicle overnight and examined after 24 h |
Data on age, sex and diet type for each cat was collected. The cats were fed a variety of canned, wet diets with or without a dry kibble (Kennelpak—Protein Plus Crunchy Mix) containing 30% protein and 10% moisture. The dry diet was mixed in with the canned food and the cats fed in groups so the relative amount of dry food that any one cat consumed was not controlled. The cats were involved in a variety of different projects. pH was measured on the initial sample using universal indicator paper and specific gravity by refractometry The sample was then centrifuged at 300 g for 5 min, 1.8 ml of supernatant removed and the sediment re-suspended in the remaining 200 μl by gentle tapping. 50 μl of stain (Kova stain—Hycor Biochemical) was added and the sample mixed by pipetting four times. One drop was transferred onto a microscope slide (Kova–Hycor Biochemical) pre-warmed to body temperature (38°C) and the sediment examined. The examination was carried out at 400× magnification and the number of struvite crystals were recorded in seven different fields in duplicate. Counts were expressed as the number of crystals per millilitre of urine. Inter and intra-assay and examiner variation was assessed but was not found to be statistically significant.
Statistical analysis was carried out using Friedman's test with Dunn post test analysis, McNemar's test, Fisher's exact test and linear regression as appropriate.
A small survey of diagnostic pathology laboratories within the United Kingdom was conducted to look at the reporting and interpretation of crystalluria on postal samples.
Results
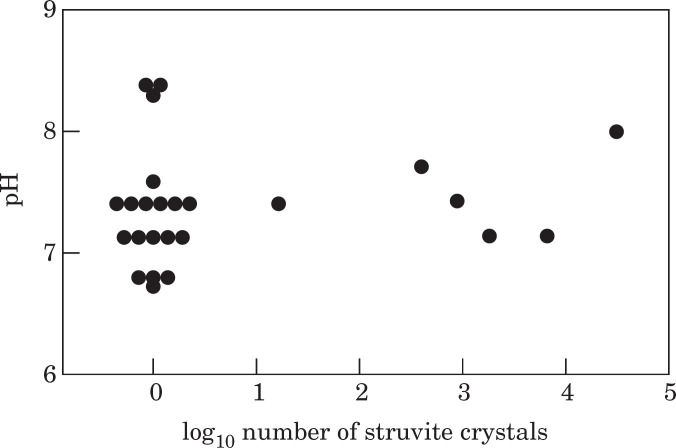
Cats ranged in age from five months to 15 years (Table 2). Thirty of the cats were considered to be of optimal bodyweight, three were considered overweight, five underweight and three unrecorded. Twenty-five were fed a mixed dry/wet diet, 16 were fed a wet diet alone. In the initial aliquot, six cats had struvite crystalluria, all were fed a mixed diet (P=0.07). In subsequent aliquots, crystalluria were never detected in cats fed all wet food but occurred in 92% of cats fed mixed diets in at least one sample (P<0.0001). Urine specific gravity was significantly higher (P<0.01) in cats fed a mixed diet (1.040 ± 0.01 vs. 1.031 ± 0.008). Cats fed a mixed diet also had a tendency to have more alkaline urine (pH 7.38 ± 0.48) compared to cats fed wet food alone (7.04 ± 0.6) but the difference was not statistically significant (P=0.06; Table 2). pH did not have a significant effect on the occurrence or magnitude of the crystalluria (Fig. 1). Age and sex of the cat was not significantly associated with urine pH, SG or the presence of crystalluria. Re-suspending and re-warming (to 38°C) the remainder from a number of samples from aliquot 3 did not result in significant re-dissolution of struvite crystals that had precipitated out during refrigeration.
Table 2.
Details of age, urine S.G. and pH
| Median | Range | 1st quartile | 3rd quartile | |
|---|---|---|---|---|
| Age (all) (months) | 21 | 5→180 | 5 | 60.75 |
| Age (wet fed) (months) | 63 | 15→180 | 21 | 77 |
| Age (mixed fed) (months) | 18 | 5→131 | 5 | 44 |
| Mean | Range | Standard deviation | |
|---|---|---|---|
| Urine SG (all) | 1.036 | 1.021→1.060 | 0.010 |
| Urine SG (wet fed) | 1.031 | 1.021→1.052 | 0.008 |
| Urine SG (mixed fed) | 1.040 | 1.025→1.060 | 0.010 |
| Urine pH (all) | 7.25 | 5.8→8.4 | 0.54 |
| Urine pH (wet fed) | 7.04 | 5.8→7.7 | 0.60 |
| Urine pH (mixed fed) | 7.38 | 6.7→8.4 | 0.48 |
Fig 1.
Effect of pH on struvite crystal numbers in cats fed a mixed dry/wet food diet.
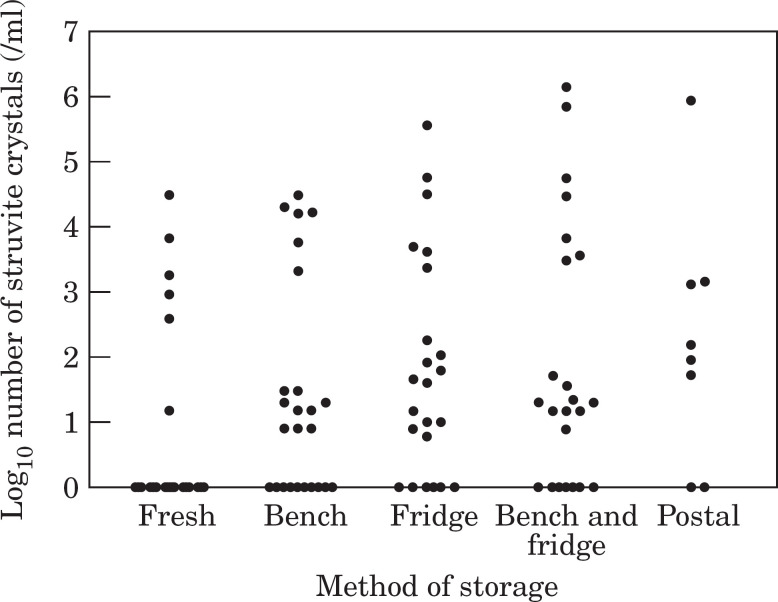
Crystalluria did not develop in any aliquots of the cats fed an all wet food diet, subsequent analysis was focused on cats fed a mixed diet. In cats fed a mixed diet, a significantly higher number of samples contained struvite crystals compared to cats fed wet food only in all aliquots. Any form of storage had a significant effect on the number of struvite crystals present in a sample (P<0.0001) (Fig. 2, Table 3). Bench storage had the least effect on the number of struvite crystals present in the aliquot compared to the fresh sample (Table 3), with bench storage followed by refrigeration having the most profound effect. Following refrigeration (aliquot 3 or 4), only one of 16 samples had a lower level of struvite crystalluria than in the fresh sample with a mean increase in the number of struvite crystals of 27,000/ml compared to an increase of 2000/ml in bench stored samples (aliquot 2). The occurrence of struvite crystals in stored urine samples (aliquots 2, 3 and 4) in cats fed a mixed diet had a specificity of between 39 and 63% and a positive predictive value of between 35% and 40% for the presence of crystalluria in a fresh sample. Sufficient urine was only obtained from 8 of 25 cats fed a mixed diet to allow a 5th aliquot (mock postage). There was a tendency for more struvite crystals to be present in more of the samples (Fig. 2) undergoing mock postage compared to the fresh sample but this was not statistical significance (P=0.25).
Fig 2.
Effect of storage on struvite crystalluria in cats fed a mixed dry/wet food diet.
Table 3.
Effect of diet and method of storage on the presence and magnitude of struvite crystalluria
| % of samples with crystalluria | Fresh | Bench | Fridge | Bench/fridge | Postage |
|---|---|---|---|---|---|
| Wet | 0 | 0 | 0 | 0 | 0 |
| Mixed | 24 | 60 * | 71 ‡ | 73 ‡ § † | 75 |
P<0.05 for the number of struvite crystals compared with analysis of fresh sample Dunn's post test.
P<0.005 for the number of struvite crystals compared with analysis of fresh sample Dunn's post test.
P<0.005 for the number of struvite crystals compared with analysis of bench sample Dunn's post test.
P<0.05 for the number of struvite crystals compared with analysis of refrigerated sample Dunn's post test.
Thirteen of 19 commercial diagnostic laboratories in the UK replied to a questionnaire on identification and clinical interpretation of crystalluria in cats. All laboratories used centrifugation to prepare samples for sediment analysis but there was a wide variation in relative force (142–8590 g) and time (3–10 min) used. Twelve laboratories examined sediment under high power, 7/13 gave a score for the number of struvite crystals the remainder gave an estimated count. 3/13 analysed the urine sample immediately on arrival, 1/13 analysed directly from the refrigerator, 3/13 refrigerated the sample and then allowed it to warm to room temperature and 6/13 stored the sample at room temperature. A report suggesting significant struvite crystalluria could mean between one and greater than 31,351 crystals per ml dependent on the laboratory involved.
Discussion
Struvite crystals were more likely to be found in the fresh urine sample of cats fed a mixed diet when compared to cats fed solely on tinned food. This is likely to be as a result of increased solute concentration (suggested by the higher urine specific gravity [Palmore et al 1978]) and the tendency to more alkaline urine (reducing the solubility [Buffington 1990, Skoch et al 1991]) in the mixed diet fed group. In this group, cooling would serve to further reduce solubility, increasing the chances of the sample becoming over-saturated leading to precipitation. If dried food constituted part of a cat's diet, the method of storage significantly altered the prevalence and magnitude of crystalluria, with refrigeration being associated with the greatest increases. This study suggests that refrigeration of urine samples (which is currently recommended [Bush 1992, Osborne et al. 1996b] if immediate analysis is not possible) should be avoided if the sediment is to be evaluated for struvite crystalluria. Due to insufficient numbers of samples, the effects of ‘mock’ postage were not fully evaluated in this study.
This study has also shown that, in stored samples, a crystalluria of greater than 1000/ml is likely to be associated with struvite crystals in the fresh sample (5/7 cases) where as a crystalluria of less than 1000/ml is unlikely to be associated with struvite crystals in the fresh sample (2/12 cases) (P<0.05, Fisher's exact test). In cats fed a mixed diet, urine pH was not found to be significantly associated with the presence of struvite crystalluria which is contrary to previously published data on post-prandial samples (Finke & Litzenberger 1992).
The data presented suggests that the diagnosis of struvite crystalluria in urine samples taken from cats fed a mixed diet is highly unreliable unless immediate analysis is undertaken. Conversely, in cats fed all wet food diets, the presence of crystalluria is likely to be a true reflection of the presence of intracystic crystals although this could not be confirmed by the study. No dry diet only group was available for inclusion, but it is likely that they would show a similar trend to cats fed a mixed diet. A recent dietary history is, therefore, crucial for an accurate interpretation of urinalysis results to be made in cats. This study further emphasises the need to consider the potential adverse effects of dietary modification before its empirical use in cats presenting with lower urinary tract signs and apparent crystalluria in the absence of a specific diagnosis.
Acknowledgements
The authors would like to thank the staff of the biochemistry laboratory at Langford for their help in providing the facilities and expertise that allowed this project to be undertaken. Thanks are also due to the research animal technicians who cared for the cats from which the urine samples were obtained, Rebecca Giles for providing the urinalysis results from the hospital database and Toby Knowles and Peter Cripps for their help and advice with the statistical analysis.
References
- Buffington CA, Rogers QR, Morris JG. (1990) Effect of diet on struvite activity product in feline urine. American Journal of Veterinary Research 51, 2025–2029. [PubMed] [Google Scholar]
- Bush B. (1992) Lower urinary tract disorders of small animals. In Practice 14, 309–316. [Google Scholar]
- DiBartola SP, Buffington CA, Chew DJ, McLoughlin MA, Sparks RA. (1993) Development of chronic renal failure in cats fed a commercial diet. Journal of American Veterinary Medical Association 202, 744–751. [PubMed] [Google Scholar]
- Finke MD, Litzenberger BA. (1992) Effect of food intake on urine pH in cats. Journal of Small Animal Practice 33, 261–265. [Google Scholar]
- Ling GV, Franti CE, Ruby AL, Johnson DL. (1990) Epizootiologic evaluation and quantitative analysis of urinary calculi from 150 cats. Journal of American Veterinary Medical Association 196, 1459–1469. [PubMed] [Google Scholar]
- Osborne CA, Thumchai R, Lulich JP, Bartges JW, Lund EM, Marsh WE, Koehler LA, Unger LK, Bird KA, St Paul MN. (1994) Epidemiology of Feline Urolithiasis. Proceedings of the 12th ACVIM Forum, San Francisco, CA, 482–483.
- Osborne CA, Kruger JM, Lulich JP. (1996a) Feline lower urinary tract disorders. Veterinary Clinics of North America: Small Animal Practice 26, 169–179. [DOI] [PubMed] [Google Scholar]
- Osborne CA, Lulich JP, Ulrich LK, Bird KA. (1996b) Feline crystalluria. Veterinary Clinics of North America: Small Animal Practice 26, 369–391. [DOI] [PubMed] [Google Scholar]
- Palmore WP, Gaskin JM, Nielson JT. (1978) Effects of diet on feline urine. Laboratory Animal Science 28, 551–555. [PubMed] [Google Scholar]
- Skoch ER, Chandler EA, Douglas GM, Richardson DP. (1991) Influence of diet on urine pH and the feline urological syndrome. Journal of Small Animal Practice 32, 413–419. [Google Scholar]