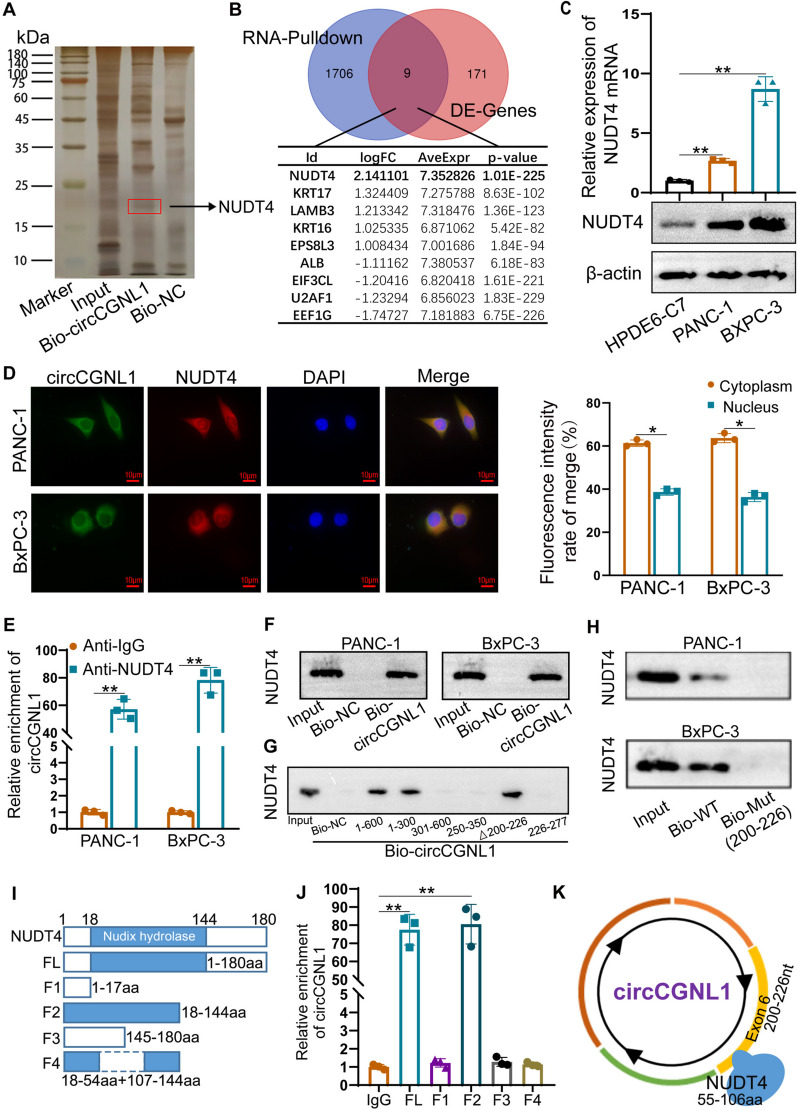
Fig. 3.
circCGNL1 physically interacts with NUDT4 protein in PC cells. A Biotinylated circCGNL1 probe (Bio-circCGNL1) and biotinylated antisense circCGNL1 probe (Bio-NC) were transcribed in vitro and incubated with protein extracts from PANC-1 cells for RNA pulldown experiments. Pulled-down proteins were harvested for silver staining, and a specific band was observed between 15 and 25 kDa (arrow). B MS and DEG analyses were performed to identify candidate proteins that could interact with circCGNL1. Nine overlapping potential binding proteins were identified and are shown. C qRT-PCR and WB assays were conducted to detect mRNA and protein expression levels of NUDT4 in PANC-1 and BxPC-3 cells. D FISH-IF assays showing that NUDT4 colocalized with circCGNL1 in PANC-1 and BxPC-3 cells. Scale bar = 10 μm. E RIP experiments were performed using antibodies against NUDT4 and IgG, and qRT-PCR was performed to detect circCGNL1 in PANC-1 and BxPC-3 cells. β-actin expression was detected as an internal control. F RNA pulldown assays further confirmed the binding between NUDT4 and circCGNL1. G Immunoblotting for NUDT4 pulled down with the circCGNL1 antisense control and different circCGNL1 truncation variants (nt 1–600, 1–300, 301–600, 250–350, 200–226, and 226–277). H Wild type (WT) and 200-226nt mutated (Mut) circCGNL1 probes were used to performed RNA pulldown assay. I, J NUDT4 protein was truncated into different fragments, including aa 1–17, 18–144 (the nudix hydrolase domain of NUDT4), 145–180, 18–54, or 107–144 (I). RIP assays were performed with the different truncated NUDT4 fragments in 293 T cells (J). K Graphic illustration of the interaction sites between circCGNL1 exon 6 and NUDT4. *p < 0.05, **p < 0.01