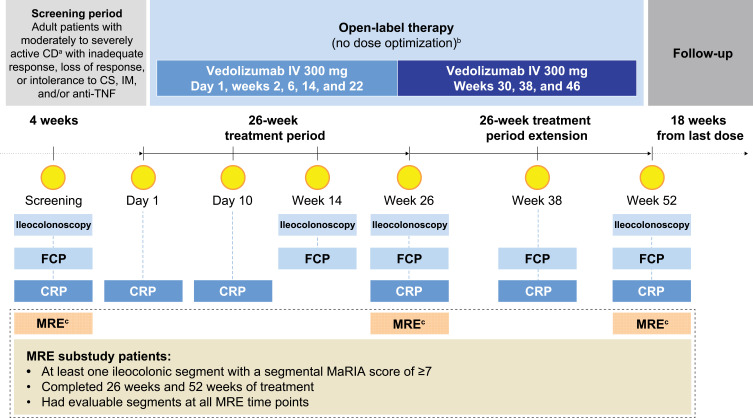
Figure 1.
Study design of the VERSIFY MRE substudy. aPatients who had a diagnosis of CD for ≥3 months and have active disease defined as a baseline CD Activity Index score of 220–450 and SES-CD of ≥7 with any ulcer (including aphthae) in any bowel segment including the ileum and/or colon documented by centrally read ileocolonoscopy. bNo option at week 10 to increase dose frequency to every 4 weeks based on clinical response (per recommended use in clinical practice). cMRE was used in a subset of 37 patients from the VERSIFY study who had baseline MRE assessments.
Abbreviations: CRP, C-reactive protein; CS, corticosteroid; FCP, fecal calprotectin; IM, immunosuppressant.