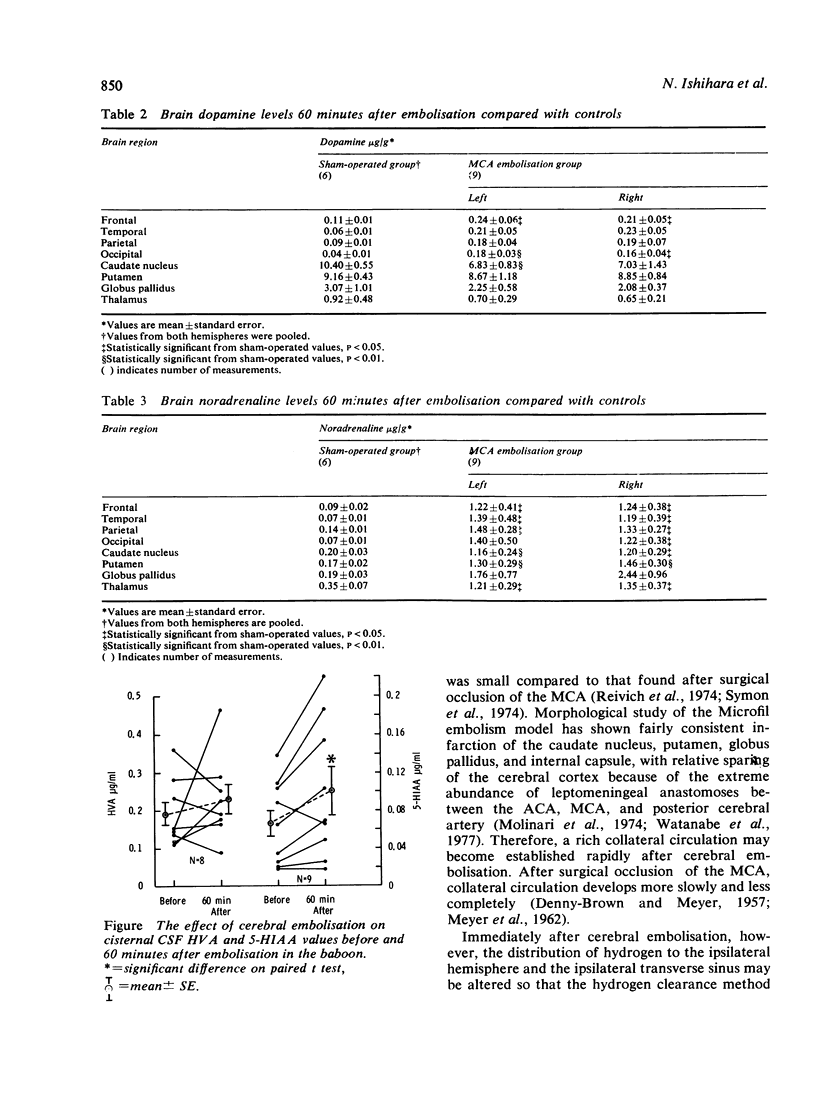
Abstract
In baboons the right cerebral hemisphere was embolised by a shower of microemboli, immediately followed by one large embolus designed to occlude the middle cerebral artery (MCA). One hour after embolism a significant, though small, reduction in blood flow and oxygen consumption of the embolised hemisphere was recorded, at which time the animals were killed and brain monoamines measured. Dopamine was reduced in the ipsilateral caudate nucleus, the reported site of maximal ischaemic damage in this model. Dopamine levels were increased in frontal and occipital grey matter sampled from areas surrounding the occluded MCA territory and in similar brain areas of the opposite non-embolised hemisphere. Noradrenaline was increased in grey matter from both cerebral hemispheres, as well as subcortical structures bilaterally. Brain 5-hydroxytryptamine levels were unaltered, but increased 5-hydroxyindoleacetic acid in cisternal cerebrospinal fluid suggested transient alteration in 5-hydroxytryptamine metabolism after embolism. The effects of cerebral embolism on brain monoamine metabolism appear to be different from the effects of permanent surgical occlusion of major cerebral vessels. The bilaterality of effects after unilateral hemispheric embolism might be related to diaschisis. The mechanisms of the observed changes, as well as their relevance to the progression of cerebral ischaemia and the complications associated with cerebral embolism, still require to be established.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. THE DISTRIBUTION OF DOPAMINE AND DOPA IN VARIOUS ANIMALS AND A METHOD FOR THEIR DETERMINATION IN DIVERSE BIOLOGICAL MATERIAL. J Pharmacol Exp Ther. 1964 Sep;145:326–336. [PubMed] [Google Scholar]
- ASHCROFT G. W., SHARMAN D. F. Drug-induced changes in the concentration of 5-OR indolyl compounds in cerebrospinal fluid and caudate nucleus. Br J Pharmacol Chemother. 1962 Aug;19:153–160. doi: 10.1111/j.1476-5381.1962.tb01436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft G. W., Crawford T. B., Dow R. C., Guldberg H. C. Homovanillic acid, 3,4-dihydroxyphenylacetic acid and 5-hydroxyindol-3-ylacetic acid in serial samples of cerebrospinal fluid from the lateral ventricle of the dog. Br J Pharmacol Chemother. 1968 Jul;33(3):441–456. doi: 10.1111/j.1476-5381.1968.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSSON A., WALDECK B. A fluorimetric method for the determination of dopamine (3-hydroxytyramine). Acta Physiol Scand. 1958 Dec 15;44(3-4):293–298. doi: 10.1111/j.1748-1716.1958.tb01628.x. [DOI] [PubMed] [Google Scholar]
- Curzon G., Green A. R. Rapid method for the determination of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in small regions of rat brain. Br J Pharmacol. 1970 Jul;39(3):653–655. doi: 10.1111/j.1476-5381.1970.tb10373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENNY-BROWN D., MEYER J. S. The cerebral collateral circulation. II. Production of cerebral infarction by ischemic anoxia and its reversibility in early stages. Neurology. 1957 Aug;7(8):567–579. doi: 10.1212/wnl.7.8.567. [DOI] [PubMed] [Google Scholar]
- Estler C. J., Ammon H. P. The influence of the beta-sympathicolytic agent propranolol on glycogenolysis and glycolysis in muscle, brain and liver of white mice. Biochem Pharmacol. 1966 Dec;15(12):2031–2035. doi: 10.1016/0006-2952(66)90231-0. [DOI] [PubMed] [Google Scholar]
- Gaudet R., Welch K. M., Chabi E., Wang T. P. Effect of transient ischemia on monoamine levels in the cerebral cortex of gerbils. J Neurochem. 1978 Apr;30(4):751–757. doi: 10.1111/j.1471-4159.1978.tb10781.x. [DOI] [PubMed] [Google Scholar]
- Gerbode F. A., Bowers M. B. Measurement of acid monoamine metabolites in human and animal cerebrospinal fluid. J Neurochem. 1968 Sep;15(9):1053–1055. doi: 10.1111/j.1471-4159.1968.tb11648.x. [DOI] [PubMed] [Google Scholar]
- Gotoh F., Meyer J. S., Ebihara S. Continous recording of human cerebral blood flow and metabolism: methods for electronic monitoring of arterial and venous gases and electrolytes. Med Res Eng. 1966;5(2):13–19. [PubMed] [Google Scholar]
- KRNJEVIC K., PHILLIS J. W. Actions of certain amines on cerebral cortical neurones. Br J Pharmacol Chemother. 1963 Jun;20:471–490. doi: 10.1111/j.1476-5381.1963.tb01484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Scheinberg P., Matsumoto A., Busto R., Reinmuth O. M. Catecholamines in experimental brain ischemia. Arch Neurol. 1975 Jan;32(1):21–24. doi: 10.1001/archneur.1975.00490430043005. [DOI] [PubMed] [Google Scholar]
- Lavyne M. H., Moskowitz M. A., Larin F., Zervas N. T., Wurtman R. J. Brain H-3-catecholamine metabolism in experimental cerebral ischemia. Neurology. 1975 May;25(5):483–485. doi: 10.1212/wnl.25.5.483. [DOI] [PubMed] [Google Scholar]
- Levy D. E., Duffy T. E. Cerebral energy metabolism during transient ischemia and recovery in the gerbil. J Neurochem. 1977 Jan;28(1):63–70. doi: 10.1111/j.1471-4159.1977.tb07709.x. [DOI] [PubMed] [Google Scholar]
- Lust W. D., Mrsulja B. B., Mrsulja B. J., Passonneau J. V., Klatzo I. Putative neurotransmitters and cyclic nucleotides in prolonged ischemia of the cerebral cortex. Brain Res. 1975 Nov 14;98(2):394–399. doi: 10.1016/0006-8993(75)90021-9. [DOI] [PubMed] [Google Scholar]
- MEYER J. S., GOTOH F., TAZAKIY Circulation and metabolism following experimental cerebral embolism. J Neuropathol Exp Neurol. 1962 Jan;21:4–24. doi: 10.1097/00005072-196201000-00002. [DOI] [PubMed] [Google Scholar]
- Molinari G. F., Moseley J. I., Laurent J. P. Segmental middle cerebral artery occlusion in primates: an experimental method requiring minimal surgery and anesthesia. Stroke. 1974 May-Jun;5(3):334–339. doi: 10.1161/01.str.5.3.334. [DOI] [PubMed] [Google Scholar]
- Renzini V., Brunori C. A., Valori C. A sensitive and specific fluorimetric method for the determination of noradrenalin and adrenalin in human plasma. Clin Chim Acta. 1970 Dec;30(3):587–594. doi: 10.1016/0009-8981(70)90249-4. [DOI] [PubMed] [Google Scholar]
- Shinohara Y., Meyer J. S., Kitamura A., Toyoda M., Ryu T. Measurement of cerebral hemispheric blood flow by intracarotid injection of hydrogen gas. Validation of the method in the monkey. Circ Res. 1969 Dec;25(6):735–745. doi: 10.1161/01.res.25.6.735. [DOI] [PubMed] [Google Scholar]
- Stone T. W., Taylor D. A. Microiontophoretic studies of the effects of cylic nucleotides on excitability of neurones in the rat cerebral cortex. J Physiol. 1977 Apr;266(3):523–543. doi: 10.1113/jphysiol.1977.sp011780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symon L., Pasztor E., Branston N. M. The distribution and density of reduced cerebral blood flow following acute middle cerebral artery occlusion: an experimental study by the technique of hydrogen clearance in baboons. Stroke. 1974 May-Jun;5(3):355–364. doi: 10.1161/01.str.5.3.355. [DOI] [PubMed] [Google Scholar]
- Valori C., Brunori C. A., Renzini V., Corea L. Improved procedure for formation of epinephrine and norepinephrine fluorophors by the trihydroxyindole reaction. Anal Biochem. 1970 Jan;33(1):158–167. doi: 10.1016/0003-2697(70)90449-5. [DOI] [PubMed] [Google Scholar]
- Watanabe O., Bremer A. M., West C. R. Experimental regional cerebral ischemia in the middle cerebral artery territory in primates. Part 1: Angio-anatomy and description of an experimental model with selective embolization of the internal carotid artery bifurcation. Stroke. 1977 Jan-Feb;8(1):61–70. doi: 10.1161/01.str.8.1.61. [DOI] [PubMed] [Google Scholar]
- Welch K. M., Chabi E., Buckingham J., Bergin B., Achar V. S., Meyer J. S. Cathecholamine and 5-hydroxytryptamine levels in ischemic brain. Influence of p-chlorophenylalanine. Stroke. 1977 May-Jun;8(3):341–346. doi: 10.1161/01.str.8.3.341. [DOI] [PubMed] [Google Scholar]
- Welch K. M., Hashi K., Meyer J. S. Cerebrovascular response to intracarotid injection of serotonin before and after middle cerebral artery occlusion. J Neurol Neurosurg Psychiatry. 1973 Oct;36(5):724–735. doi: 10.1136/jnnp.36.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch K. M., Meyer J. S., Teraura T., Hashi K., Shinmaru S. Ischemic anoxia and cerebral serotonin levels. J Neurol Sci. 1972 May;16(1):85–92. doi: 10.1016/0022-510x(72)90103-7. [DOI] [PubMed] [Google Scholar]
- Welch K. M., Wang T. P., Chabi E. Ischemia-induced seizures and cortical monoamine levels. Ann Neurol. 1978 Feb;3(2):152–155. doi: 10.1002/ana.410030211. [DOI] [PubMed] [Google Scholar]
- Zervas N. T., Hori H., Negora M., Wurtman R. J., Larin F., Lavyne M. H. Reduction in brain dopamine following experimental cerebral ischaemia. Nature. 1974 Feb 1;247(5439):283–284. doi: 10.1038/247283a0. [DOI] [PubMed] [Google Scholar]


