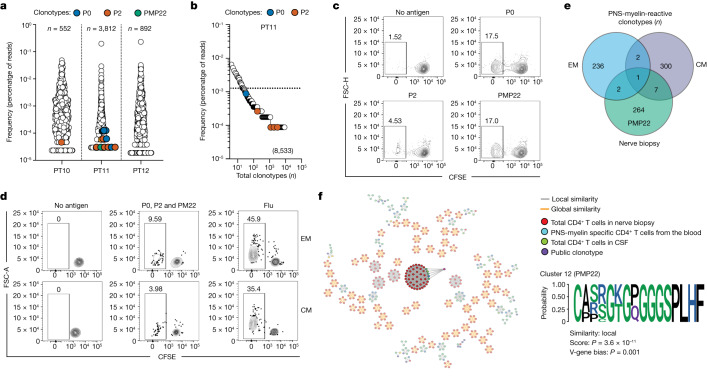
Fig. 5. Identification of autoreactive CD4+ T cells in the CSF and peripheral nerves of patients with GBS.
a,b, TCR Vβ sequencing was performed on in vitro expanded CD4+ T cells sorted from the CSF (a) or ex vivo sorted memory CD4+ T cells from the blood (b). TCRβ clonotype frequency distributions in the CSF (a) or the peripheral blood (b) of patients with GBS are shown as the percentage of reads. Coloured circles represent autoreactive TCRβ clonotypes (blue, P0-specific; orange, P2-specific; green, PMP22-specific). Values represent the total number of TCRβ clonotypes found in the CSF or blood. c,d, Total CD4+ T cells isolated from in vitro expanded T cells from a peripheral nerve biopsy (c) or EM and CM CD4+ T cells directly isolated from the blood (d) of one patient with GBS (PT16) were in vitro stimulated with autologous monocytes in the presence or absence of P0, P2 and PMP22 myelin peptide pools separately (c) or a mixture of P0, P2 and PMP22 peptide pools or influenza vaccine (Flu) as a positive control (d). Shown are the CFSE profiles of the total CD4+ T cells from the nerve biopsy (c) or of the EM and CM CD4+ T cells from the blood (d). e, Comparison of the TCRβ clonotype compositions of PNS-myelin-reactive CFSElow cells in EM and CM CD4+ T cells from the blood and PMP22-myelin-reactive CFSElow T cells from the nerve biopsy, identifying 10 unique TCRβ clonotypes shared between nerve tissue and blood. f, GLIPH2 graph showing identified paratope hotspots. Cluster 12 is highlighted and the specificity and consensus amino acid sequence are reported.