Abstract
Success in speed swimming depends on the efficiency of the anaerobic system for the production of cellular energy, especially during muscle power production. In the adolescent athletes much is unknown with regards to the relationships between relative power of upper and lower limbs with speed swimming performance. The aim the present study was to identify differences in relative muscle power of upper and lower limbs in adolescent swimmers and relate these to speed swimming performances. Sixty adolescents, of both sexes (50% female, 50% male, 30 swimmers and 30 controls), were recruited. The relative upper limb power (ULP[W/kg]) was assessed by a medicine ball test and the relative lower limbs power (LLP[W/kg]) by a jump test on a jumping platform. Lean mass of the upper and lower limbs was assessed by dual-energy X-ray absorptiometry (DXA) (g). Sport performance was assessed during national level competition (50-m swimming time [in seconds]). Biological maturation (BM) was indexed by years from attainment of peak height velocity. ULP(W/kg) was higher than LLP(W/kg) in both groups (p < 0.05). Upper and lower limb lean mass (g) correlated significantly with ULP(W/kg) and LLP(W/kg) in both groups (p < 0.05). ULP(W/kg) and LLP(W/kg) correlated with 50-m swimming performance (s), in both sexes (p < 0.05). Advanced BM was associated with ULP(W/kg) and LLP(W/kg) in both groups (p < 0.05), and with 50-m swimming performance (s) in both sexes (p < 0.05). We concluded that ULP(W/kg) is higher than LLP (W/kg) in adolescent swimmers. Upper and lower limb lean mass and BM were both positively associated with increased ULP (W/kg) and LLP (W/kg).
Keywords: Performance, Sport, Swimming, Biological maturation
Graphical abstract
List of abbreviations & acronyms
- ATP-CPr
Adenosine triphosphate- Creatine phosphate
- BM
Biological maturation.95%
- CI
Confidence interval of 95%
- °C
Temperature in degrees Celcius
- cm
Centimeters
- DR
Distance reach
- DXA
Dual-energy X-ray absorptiometry
- GH
Growth hormone
- IGF-1
Insulin-like growth factor 1
- IQR
Interquartile Range
- ISAK
International Society of the Advancement of Kinanthropometry
- kg
Kilogram
- LLP
Lower limbs power
- m
Meters
- n
Absolut number
- PHV
Peak height velocity
- r
Correlation coefficient
- RN
Rio Grande do Norte
- s
Seconds
- STROBE
STrengthening the Reporting of OBservational studies in Epidemiology
- TF
Time flying
- ULP
Upper limbs power
- W/kg
Watts per kilograms (relative power)
- %
Percentage
- ∗
Statistical significance (p < 0.05)
1. Introduction
Swimming is the self-propulsion (power) of a person through a liquid medium. However, there is no consensus about which body segment (i.e., upper and lower) provides the greatest contribution to total power.1, 2, 3 Swimming power is important for swimming performance.2,4 Competitors swim different distances in different levels of competition; including events from 50 m to 1 500 m in length. During a competitive swimming speed race (i.e., 50 m over durations ranging from 20 s to 35 s), the body primarily resorts to the anaerobic (i.e. non-oxidative) energy pathways.5 Understanding the differences between upper and lower limb anaerobic muscle power in swimmers will aid in understanding swimming power development and the interaction between glycolytic energy systems with sport performance.
When assessing type II muscle fibres (i.e., speed fibres), the anaerobic muscle power produced during fast activities (i.e., durations of one to 7 s) may be an indirect indicator of the efficiency of the anaerobic (especially ATP-CPr) system.6,7 Anaerobic muscle power is defined as the ability to produce force and speed in a short period of time, measured in watts.8,9 In swimming, as in other sports (i.e. combat sports, athletics, etc.) anaerobic muscle power is one of the main variables in determining performance success.1,10,11 It is systematically developed during daily training routines in athletes regardless of competitive level or age group.12 This is particularly true for adolescent swimmers competing in short races of distances between 50-m and 200-m.11,13
Previously in adolescent athletes it was identified that biological maturation, body morphology, and the predominance of type I or II fibers were all related to anaerobic muscle power development.14,15 For example, researchers have found that relative upper limb power (ULP) is higher than relative lower limb power (LLP) in adolescents of average and late maturity.16 This suggests that the upper limbs have a higher anaerobic capacity than the lower limbs and this is maturity dependent. However, the specificity of swimming16 was not analyzed; therefore, it is of interest to investigate the differences between ULP and LLP in adolescent sprinter swimmers.
Anaerobic power can be estimated by means of effort tests such as the Wingate test, which can be performed for both upper and lower limbs separately.17,18 However, this type of estimation requires the use of sophisticated equipment and must be performed by trained professional familiar with the method. In the non-scientific setting of swimming clubs’ alternative assessments are required. It has been shown that swimming coaches can analyze muscle power through simple and accessible tests, such as the medicine ball throw test17,19 for ULP and the vertical jump test20,21 for LLP. Another component that has indicated significant interactions with swimming performance is body morphology22; however, information on the relationships of upper and lower limb morphology with speed swimming performance is still inconclusive.
The primary objective of this study was to compare anaerobic muscle power development in the upper and lower limbs of adolescent swimmers. The secondary objectives were to verify the relationship of upper and lower limb morphology and sport performance in a 50-m swim with ULP and LLP muscle power. The study also investigated the relationship between biological maturation, ULP and LLP, and sport performance. It was hypothesized that there would be significant differences between ULP and LLP in adolescent swimmers in both sexes.
2. Methods
A total of 30 competitives23 swimmers (average age: [13.8 ± 1.4] years old [50% male and 50% female]) were recruited. Participants had been exposed to systamatic training for between 3 and 7 years. Thirty adolecent controls (average age: [14.5 ± 1.0] years [50% male and 50% female]) were also recruited. The sample size was determined a priori using the statistical software G∗Power (Version 3.1, Düsseldorf, Germany) with the configuration "T" family tests for dependent means (Wilcoxon test). The effect size identified by Almeida-Neto et al.16 was used to compare the relative power in watts of the upper limbs versus the lower limbs in adolescents of different maturational timings (early, average and late). We considered standard values of α = 0.05 and β = 0.8 and arrived at a minimum sample size of 10 subjects per group (critical t > 1.8, sample power > 0.8).
To recruit the sample, we contacted the swimming federation of Natal (RN, Brazil). The federation provided information of the swim teams in the city that had adolescent athletes. Four teams who annually participated in national level competitions were identified and the coaches contacted. Subsequently, adolescent athletes, and their guardians were provided with the study information package. The following inclusion criteria for those interested in participating was used: (i) participation in physical education practices at school, (ii) involvment in a systematic training regime (> 3 h per day, > 4 days per week) (iii) membership of a major sports clubs, and (iv) participation at national and/or international competition level. Athletes with any neuromotor limitation or who were consuming exogenous substances (e.g., creatine, caffeine & taurine) were excluded.
The control group was recruited from a social project aimed at the recreation of children and adolescents; the participants of the social project participated in group games twice a week. For the control group, the inclusion criterion was participation in school physical education and exclusion criteria were performing in a systematized sport and presenting with a musculoskeletal injury.
2.1. Ethical approval
This study was approved by the Ethical Committee of the Federal University of Rio Grande do Norte (#4.236.385) in Rio Grande do Norte state, Brazil, in accordance with Resolution 466/12 of December 12, 2012, of the National Health Council and the Helsinki Declaration.24 Reports were prepared following the STROBE statement.25 All participants and their respective guardians signed the consent or assent forms before participating in this research. Therefore, by signing the consent forms, this research obtained formal consent from the participants and their guardians. The protocol of the present study was registered a priori and is publicly available on the Open Science Framework Registries platform (DOI: 10.17605/OSF.IO/DU96X).
2.2. Procedures
Participants were initially informed about the risks and benefits of the procedures before agreeing with the consent terms. Thereafter, the anthropometric data was collected by a researcher blinded to the participant grouping. Twenty-four hours prior to the relative upper limbs power (ULP) and relative lower limbs power (LLP) tests. After one week the national level swimming competition took place, and sports performance was assessed during a 50-m swim (race time in seconds).
2.3. Blinding
The evaluators who performed the tests in the laboratory (anthropometry, ULP and LLP) had no knowledge about which group the participant belonged to (Swimming or Control), in addition the evaluators and participants were blinded in relation to the biological maturation stages of the individual. The sport performance was analyzed by mediating the sport competition result, this guaranteed the blinding of the evaluators preventing them from interfering in a positive or negative way in the swimmers' performance. For the treatment of data involving maturation, body composition, ULP and LLP, we masked the identifications of the groups and the stage of biological maturation. Finally, the data treatment involving sport performance was performed by an external researcher to the research.
2.4. Randomization
The order of the ULP and LLP tests were randomly assigned on the heads or tails toss of a coin.
2.5. Anthropometry
The anthropometric evaluations were performed with participants barefooted and wearing only light clothing. Body mass was measured using a Filizola® (São Paulo, Brazil) digital scale (with a capacity up to 150 kg and a variation of 0.10 kg); stature was measured using, the Sanny® stadiometer (São Paulo, Brazil) (0.1 mm accuracy). All measurements were taken following the International Society of the Advancement of Kinanthropometry (ISAK) protocols.26 For the technical error of intra-examining anthropometric measurements, the following magnitude was used27: acceptable ≤ 1.0%. Body composition was assessed by dual-energy X-ray absorptiometry (DXA). The technician was a member of the research team with prior training (LUNAR®/GE PRODIGY - LNR 41,990, Washington, DC, United States), this procedure is considered one of the most reliable standards for measuring body composition.28 Through the use of appropriate algorithms for the pediatric population (GeLunar ®, V.15.00, Washington, DC, United States), DXA analysis provided data on fat mass and lean mass in grams.28 DXA used the following standardization during the evaluations: Full Body Evaluation, Voltage (kV): 76.0, Current (mA): 0.150, Radiation dose (μGγ): 0.4 (Very low, no health risk).
2.6. Biological maturation
Biological maturation was expressed as a biological age; years from the attainment of Peak Height Velocity (PHV), termed maturity offset, was estimated from chronological age and stature. At 0.00 years PHV was being attained; PHV is a measure of somatic maturation. Years from PHV was predicted using mathematical models proposed by Moore et al.29 (for boys between 8 and 18 years old, and girls between 8 and 16 years old):
| Maturity offset in males = −7.999 994 + [0.003 612 4 × (Age (years) × Stature (cm))] |
| Maturity offset in females = −7.709 133 + [0.004 223 2 × (Age (years) × Stature (cm))] |
(cm) centimeters.
Participants were classified as pre-PHV (maturity offset < −1.00 years from the average PHV) circum-PHV (between −1.00 and 1.00 years from the average PHV), and post-PHV (> 1.00 years from the average PHV).29
2.7. Upper limbs power
Participants were instructed to restrict physical efforts for 24 h prior to testing. For the test, participants were seated on the floor with their backs sustained against a wall with 90° hips flexion and extended knees. They then threw a 2-kg medicine ball (Ax Esportes®, Tangará, Brasil horizontally from their sternum, using both hands and without assistance of the trunk.19 Three attempts, with 90-s interval, were asssessed and the furthest distance thrown was recorded. The time of flying (TF) and the distance reached (DR) by the medicine ball were registered. Relative upper limbs power (ULP) was calculated on the basis of Newtonian physics as30: Relative power (W/kg) = (Medicine ball mass [kg] × DR [m])/TF (s).
2.8. Lower limbs power
The relative lower limbs power (LLP) was determined by a countermovement jump test, using a platform with interruption system (CEFISE®, São Paulo, Brazil) following the protocol established by Bosco et al.20 The participants first performed one practice jump to reduce errors during the protocol execution. Thereafter, from holding an orthostatic position for 3 s with the knees flexed at 90° and hands fixed on their waist, they first performed asquat followed by a maximum effort jump. Three tests’ attempts interspersed with 90-s of passive recovery were executed and the best score was recorded. Data was used to determinate the relative power (W/kg).
2.9. Sport performance
Sport performance was assessed during national level competition. The competition took place in an Olympic-sized swimming pool (50-m long, 25-m wide, 2.5-m deep, treated with chlorine, water temperature = 24 °C, room temperature = 27 °C) located in a sports club in the city of Natal/Brazil. At the end of the competition, after the official rankings were published, result of the main official competitive swimming style of each athlete was complied as swim times in seconds. The distribution of the athletes among the swimming tests in competition was as follows: 50-m Backstroke (5 males and 5 females); 50-m Burtterfly (5 males and 5 females); 50-m Crawl (5 males and 5 females).
2.10. Statistic
The normality of the data was tested using a Shapiro-Wilk and Z-score tests for skewness and kurtosis (−1.96 to 1.96). Comparisons were performed using the Wilcoxon test. Effect size between the differences was verified by Cohen's d test, being interpreted by the magnitude31: Small < 0.20; Medium > 0.20 and < 0.50; Large > 0.50. Correlations were performed using Spearman's test and magnitude identified using criteria proposed by Schober, Boer & Schwarte32: Insignificant: r < 0.10; Weak: r = 0.10–0.39; Moderate: r = 0.40–0.69; Strong: r = 0.70–0.89; Very strong: r = 0.90–1.00. For the linear regression analyses, the data were log transformed to base 10. For the correlations, we analyzed whether the development of biological maturation (years from PHV) was associated with increase in muscle power and lean mass of the upper and lower limbs. All analyses were performed using open source software JASP® (version 0.15.0.0; University of Amsterdam, Holland) considering p < 0.05. All figures and graphical analyses were performed in GraphPad Prism software (Version 8.01 244, California, USA).
3. Results
The majority of male subjects were predicted to be circum-PHV and female subjects predicted to be post-PHV (p < 0.05) (See Table 1). When considering body morphology, male subjects showed lower adiposity and higher concentrations of lean mass compared to their female counterparts (p < 0.05). As well, muscle power was higher in males regardless of body segment (p < 0.05). Compared to the control group, for both sexes the swimmers showed lower levels of fat (p = 0.02) and higher concentrations of lean mass in the upper (p = 0.01) and lower (p = 0.04) limbs.
Table 1.
Sample characterization.
| Variables | Swimming |
Control |
||
|---|---|---|---|---|
| Male Sex (n: 15) |
Female Sex (n: 15) |
Male Sex (n: 15) |
Female Sex (n: 15) |
|
| Median (IQR) | ||||
| Chronological age (years) | 13.8 (2.0) | 13.8 (2.7) | 15.1 (0.6) | 14.5 (1.6) |
| Biological maturation (PHV) | 0.5 (1.3) | 1.3 (1.6) | −0.2 (1.3) | 1.2 (1.5) |
| Stature (cm) | 169.0 (6.3) | 156.7 (8.0) | 171.0 (8.0) | 164.5 (8.1) |
| Wight (kg) | 54.2 (13.1) | 49.0 (13.6) | 57.4 (8.7) | 54.5 (26.3) |
| Fat mass (kg) | 8.7 (2.7) | 13.0 (5.7) | 11.0 (3.4) | 14.8 (10.7) |
| Total lean mass (kg) | 44.5 (13.8) | 34.0 (7.3) | 46.7 (8.5) | 35.0 (5.1) |
| Arm lean mass (kg) | 5.8 (1.8) | 4.3 (0.9) | 4.6 (0.8) | 3.5 (0.5) |
| Leg lean mass (kg) | 14.7 (4.5) | 11.1 (2.3) | 14.0 (2.5) | 12.2 (1.8) |
| Sport performance in swim 50-m (S) | 34.4 (1.8) | 36.0 (2.2) | Not applicable | Not applicable |
n: Absolut number. (IQR): Interquartile Range. (PHV): Peak Height velocity. (cm): Centimeter. (kg): kilograms. -m: Meters. (S): Second.
Regardless of group or sex (males’ Fig. 1 A and C; females Fig. 1 B and D) ULP was greater than LLP (Swimming male sex - Effect Size: 2.2, 95% CI: 1.2; 3.1. Swimming female sex - Effect Size: 0.9, 95% CI: 0.8; 1.0. Control male sex - Effect Size: 2.9, 95% CI: 1.7; 4.1. Control female sex – Effect Size: 2.5, 95% CI: 1.5; 3.6).
Fig. 1.
Comparison of upper and lower limbs power. Fig. 1 A: Swimming male sample. Fig. 1 B: Swimming female sample. Fig. 1C: Control male sample. Fig. 1 D: Control female sample. ULP: Upper limbs power. LLP: Lower limbs power. Kg: Kilogram. ∗: Statistical significance.
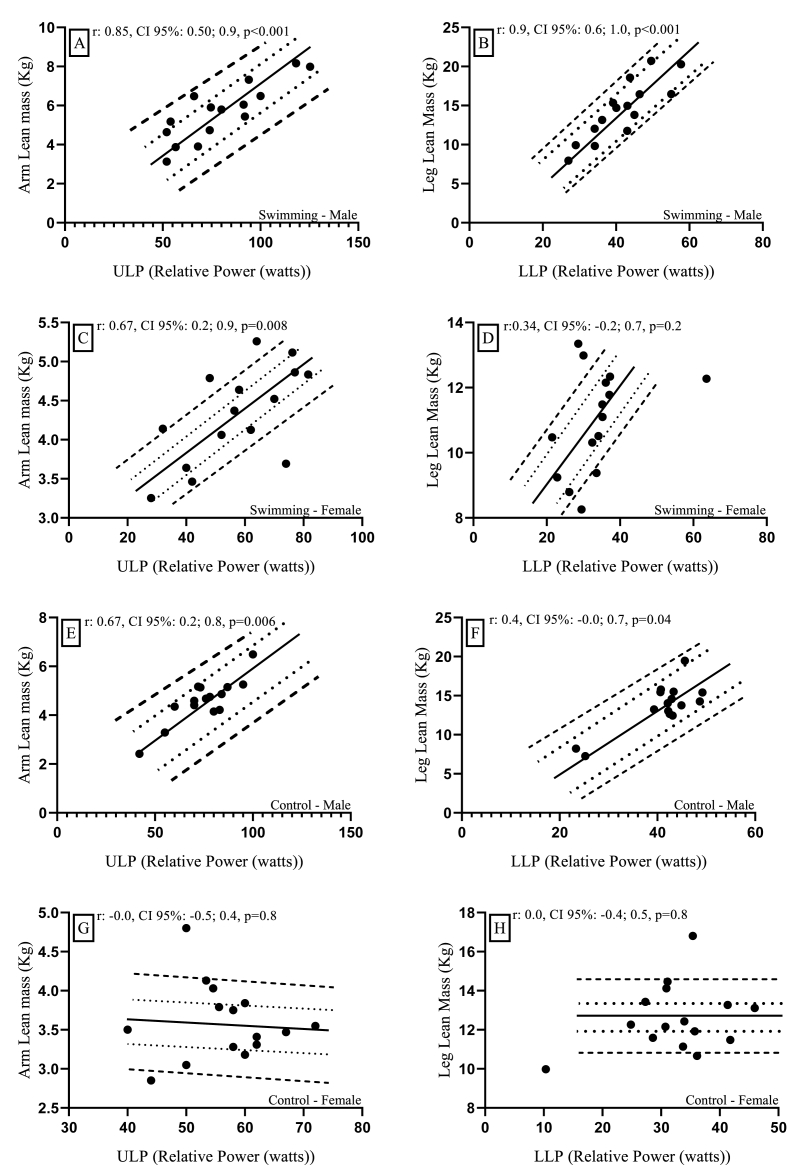
In male swimmers, upper limb lean mass and lower limb lean mass correlated with ULP and LLP. Between 72% and 73% of ULP and LLP respectively (ULP: p < 0.001. LLP: p < 0.001) (See Fig. 2 A and B). In female swimmers, the lean mass of the lower limbs showed a contribution of 41% to ULP (p = 0.1) (See Fig. 2C). However, the lean mass of the lower limbs did not show a significant correlation LLP (p = 0.7) (See Fig. 2 D).
Fig. 2.
Correlation and regression of upper and lower limbs lean mass with upper and lower limbs power. Fig. 2 A, B: Swimming male sample. Figure C, D: Swimming female sample. Figure E, F: Control male sample. Figure G, H: Control female sample. r: Correlation coefficient. 95% CI: Confidence interval of 95%. ULP: Upper limbs power. LLP: Lower limbs power. ---- internes: 95% CI correlation. ---- externals: 95% CI regression. (s): Seconds. (kg): Kilogram.
For the male control group, upper limb lean mass contributed 44% to ULP (p < 0.001), while lower limb lean mass indicated a contribution of only 16% to LLP (p < 0.001) (See Fig. 2 E and F). For females no significant correlations of upper and lower limb lean mass were found for ULP and LLP respectively (See figure G and H).
In male swimmers, ULP indicated a 98% contribution (p < 0.001) and LLP a 53% contribution (p > 0.001) with 50-m swim performance (see Fig. 3 A and B). In addition, lean arm and leg mass made contributions of 70% and 64% to 50-m swimming performance, respectively (Arm: p < 0.001. Leg: p < 0.001) (See Fig. 3C and D). For female swimmers, ULP indicated a contribution of 82% (p < 0.001) to sport performance, while LLP did not show a significant correlation (p = 0.2) (See Fig. 3 E and F). Upper and lower limb lean mass made contributions between 44% and 49% to 50-m swimming performance, respectively (Arm: p = 0.006. Leg: p = 0.006) (See Fig. 3 G and H).
Fig. 3.
Correlation and regression of upper and lower limb power and lean mass with 50-m swimming sport performance. Fig. 3 A, B, C and D: Swimming male sample. Fig. 3 E, F, G, and H: Swimming female sample. r: Correlation coefficient. 95% CI: Confidence interval of 95%. ULP: Upper limbs power. LLP: Lower limbs power. ---- internes: 95% CI correlation. ---- externals: 95% CI regression. (s): Second's. (kg): Kilogram.
Regardless of the group or sex of the subject, advanced stages of biological maturation were associated with increased: muscle power (Fig. 4 A, p < 0.05), lean mass of the upper and lower limbs (Fig. 4 B, p < 0.05), and increased swimming performance (Fig. 4 C, p < 0.05). The separate results by gender and by group can be seen in supplementary file 1 (Table 1).
Fig. 4.
Analyses of the association of upper and lower limb power, and upper and lower limb lean mass levels with advancing biological maturation. Fig. 4 A: Total sample - correlation of upper and lower limb power with height velocity peak. Fig. 4 B: Total sample - correlation of upper and lower limb lean mass with peak height velocity. Fig. 4C: Correlation of swimmers' performance with peak height velocity. (kg): Kilograms. ULP: Upper limbs power (W/kg). LLP: Lower limbs power (W/kg). PHV: Peak Height Velocity.
4. Discussion
The primary objective of this study was to compare relative anaerobic muscle power development between the upper and lower limbs in adolescent swimmers of both sexes. Our initial hypothesis was that there would be significant differences between the relative power produced by the upper and lower limbs. The findings of the present study confirmed this hypothesis.
4.1. Differences between upper and lower limbs
The results of the present study suggest that muscle power production in a ∼3-s to 4-s duration stimulus (ATP-CPr energy production system) is more efficient in the upper limbs compared to the lower limbs, regardless of the sex or biological maturation of the swimmer. These characteristics can be justified due to the predominant muscle fiber type in upper and lower limb muscles. At the superficial level the biceps brachii and triceps brachii show a higher level of type II fibers, and at the deep level a higher level of type II fibers remains for the triceps, while for the biceps, type I fibers are in higher number. For the biceps femoris at the superficial level the predominance was of type II fibers and at the deep level of type I fibers, while for the gastrocnemius and soleus there is predominance of type I fibers both superficially and deeply.33
In studies conducted by Van Hall et al.34 and Calbet et al.35 they made direct comparisons between the metabolisms of the upper and lower limb muscles, both studies indicated that regardless of the level of training the upper limb muscles are less oxidative compared to the lower limbs, indicating greater variability in blood flow during exercise. Subsequently, Helge36 found that performing exercises with the upper limbs has a lower fat oxidation compared to those of the lower limbs, suggesting glycolytic predominance in the upper limbs compared to the lower limbs.
Ørtenblad et al.37 analyzed the metabolic differences and predominance of muscle fiber types in the triceps brachii and vastus lateralis thigh muscles in elite adult skiing athletes. It was suggested that the lower limbs pointed to a higher oxidative capacity due to the percentage of 3-hydroxy-acyl-CoA dehydrogenase capacity being higher in the leg muscles. In addition, the authors found similar aerobic capacity was found in type I and IIa fibers regardless of whether the muscle was in the upper or lower limb. Thus, the findings of the present study are supported by the fact that the upper limbs are likely to have a predominance of type II muscle fibers compared to the lower limbs, which implies an increase in the efficiency of the anaerobic system in the upper body segment, which contributes to a better efficiency in producing muscle power.
Regarding adolescent athletes, Almeida-Neto et al.16 showed in both sexes, that different stages of biological maturation were linked to differences between ULP and LLP produced during activities of average duration from 3 s to 4 s. The authors found that at late and average skeletal ages stages ULP levels were higher than LLP levels, and that in early skeletal age stage this difference did not exist, suggesting that as maturation advanced the efficiency of upper and lower limbs in response to short duration and high velocity stimuli, were similar. Almeida-Neto et al.16 did not consider the specificity of the athletes' sport practice in their study, however, the present study analyzed only swimmers and found similar results.
4.2. Biological maturation
The results of the present study also suggest that in adolescent swimmers, of the same chronological age but who are more advanced in biological maturation, the production of anaerobic muscle power in the upper and lower limbs is higher compared to their chronological aged peers who are less mature. In this sense, the different stages of biological maturation have distinct characteristics regarding the physiology of anaerobic muscle power production.16,38
According to Ratel & Blazevich,39 during the late stages of maturation due to the body of young athletes being smaller in global size, the favoring of large blood circulation occurs, thus gas exchange between the muscles and the lungs happens more rapidly. This is because metabolically the pathway that oxygen takes for energy production becomes shorter in late maturing subjects (due to small body global size), which increases the efficiency of the oxidative system compared to the glycolytic.40
Birat et al.41 confirmed Ratel & Blazevich39 findings by identifying that the oxidative profile of late maturing youth was similar to that of elite endurance running athletes. Using biopsies Evangelista et al.42 analyzed the muscle fiber morphology of the deltoid muscle in young aged 0–18 years. Type I muscle fibers is greater than type II, and with the advance of puberty the type II muscle fibers stand out in relation to type I.
Previously it was observed by Kaczor et al.43 that with advancing maturity there is an increase in anaerobic enzymes (i.e., lactate dehydrogenase, creatine kinase and adenylate kinase) and a reduction in aerobic enzymes (i.e., carnitine palmitoyltransferase and 2-oxoglutarate dehydrogenase). It is conjectured that this may be related to the increased efficiency of the anaerobic ATP-CPr system and consequently to the increased efficiency of muscle power production.
In addition, in a pioneering study using magnetic resonance analyses, Neu et al.44 found that compared to their peers with advanced maturation, the forearm musculature is smaller and less voluminous in subjects with late maturation. This is justified on the basis of endocrine changes that occur with advancing biological maturation.45,46 In the advanced stages of maturation, peak discharges of androgenic hormones such as testosterone in males and estradiol in females occur.47 Peaks of growth hormone (GH) and insulin-like growth factor 1 (IGF-1), which are important regulators of the development of muscle mass volume, also occur in both sexes.48
Considering that the ATP-CPr and glycogen reserves are in the muscle cells, it is natural that a less voluminous musculature has lower reserves of these compounds.12,49 This corroborates one of the secondary findings of the present study, where a relationship was found between the increase in lean mass of the upper and lower limbs with the increase in ULP and LLP, respectively. Secondary results of the present study also indicated that sport performance in 50-m swimming increases with advancing biological maturation.
Considering advanced stages of biological maturation have a higher efficiency of the anaerobic energy production system compared to their peers in late stages of maturation,40 and that a 50-m stimulus in swimming uses in the initial phases of the race (first 10 m) the ATP-CPr system and in the rest of the race muscle glycogen (> 10 m–50 m) for energy production,50 it is justifiable that athletes with advanced maturation are more efficient in speed swimming competitions.
4.3. Muscle power and performance in sprint swimmers
The present study identified that regardless of the swimming style (crawl, breaststroke, or butterfly) the 50-m swimming performance in sports competition is associated with the levels of ULP, LLP, and upper and lower limb lean mass. Because swimming's main objective is to cross the liquid medium in the shortest possible time, it is necessary to achieve high levels of swimming propulsion which is related to ULP and LLP.51,52 This is visible when comparing elite athletes (i.e., national and international levels) with recreational practitioners of the sport, the elite level shows higher levels of ULP and LLP.53
To have an efficient swimming propulsion, the athlete depends on technical performance and biomechanics that influence the coordination of the upper and lower limbs and consequently the production of ULP and LLP.54,55 Kováčová & Broďáni,56 highlight that the lower limbs favor the locomotion of swimmers in the aquatic environment. Apparently, due to the horizontal position of the body during swimming, when moving the lower limbs there is a greater contribution of LLP to the athlete's glide, favoring the increase in speed.57
Using an allometric model, Dos Santos et al.22 found that in adolescent swimmers of both sexes, upper limb propulsion and body morphology with lower adiposity were the main determinants for 50-m performance in crawl style swimming. Previously, in a study by our group it was found that in adolescent swim competitors at the national level, lean mass levels were associated with ULP and LLP,58 ULP and LLP levels are known to be associated with swim propulsion.53,59
Based on the present discussion, it can be seen that although there is no consensus on the contribution of upper and lower limbs to 50-m swimming performance, there is convergence that muscle power and body morphology with a predominance of lean mass over fat mass are essential for the performance of sprinter athletes in swimming.
4.4. Limitation and suggestions for new studies
Although the findings of the present study are significant, our main limitation is the fact that the study design did not allow us to establish cause and effect, just associations. Moreover, we inferred the efficiency of anaerobic muscle power production by tests that demanded a high intensity muscle contraction in a short period of time, being absent the biochemical analyses to verify the predominance of anaerobic enzymes in the upper and lower limbs and associate them to ULP and LLP, respectively.
We suggest that future studies verify experimentally and longitudinally the increase in ULP and LLP levels in adolescent swimmers and seek to verify the influence of this development on sport competition results. We also recommend monitoring body morphology and biological maturation stages. Finally, we recommend that future studies use laboratory analyses (i.e., biochemistry) of alactic and lactic anaerobic metabolism (glycogen and ATP-CPr), together with anaerobic capacity tests such as the wingate test for upper and lower limbs, and that muscle activation (surface electromyography) of the main muscles involved in the wingate tests be analyzed as control variables, as well as ultrasound or magnetic resonance imaging of the same muscles. Thus, it will be possible to verify how the muscles activate during the use of the ULP and LLP and how the muscle size relates to the anaerobic performance of the upper and lower limbs.
4.5. Practical applicability suggestion
As practical application, we suggest that swimming coaches consider the stage of biological maturation to designate the events in which athletes should compete. Athletes in the pre-PHV stage indicate a better efficiency for oxidative energy production, which may favor their performance in endurance races. In the same sense, athletes in circum-PHV stage may have more advantages in longer speed events like the 200-m and 400-m swim, while athletes in post-PHV stage will have more advantages in pure speed events (50 m and 100 m). Regarding sports training, we suggest that lower limb power be worked with greater emphasis on athletes competing in speed trials, regardless of their stage of biological maturation. In addition, we suggest that sports professionals try to perform specific work in water and work on land, such as sprints on land and plyometric training for lower and upper limbs.
5. Conclusion
We conclude that in adolescent national-level swimmers, relative anaerobic muscle power is greater in the upper limbs compared to the lower limbs. We further conclude that upper and lower limb lean mass are associated with increased anaerobic muscle power in the upper and lower limbs, and these are associated with increased sport performance in 50-m swimming competition. Finally, we conclude that advancing biological maturation is associated with increased levels of lean mass, relative anaerobic muscle power, and consequently with 50-m swimming sport performance, suggesting that maturation is a variable that should be considered for training, mentoring, and selection of young talent in swimming.
Submission statement
The authors declare that they have not submitted the manuscript "Are there differences in anaerobic relative muscle power between upper and lower limbs in adolescent swimmers: A blinded study" to any journal other than Sports Medicine and Health Science. In addition, all authors have read and agree with manuscript content, the manuscript will not be submitted elsewhere for review and publication.
Authors' contributions
Paulo Almeida-Neto - Responsible for the concept/design, the data collection, the data analysis/interpretation and drafting the article. Adam Baxter-Jones – Responsible for translate to English, drafting the article and critical revision of the article. Jason Medeiros - Responsible for data collection, drafting the article and critical revision of the article. Breno Cabral – Responsible for the concept/design, project supervision, the data collection, drafting the article and critical revision of the article. Paulo Dantas - Responsible for project supervision, data analysis/interpretation and drafting the article. All authors have read and agree with manuscript content.
Ethical approval statement
This study has the approval of the Ethical Committee of the Federal University of Rio Grande do Norte (#4.236.385) in Rio Grande do Norte state, Brazil. All participants and their respective guardians signed the consent or assent forms before participating in this research. Therefore, by signing the forms, this research obtained the formal consent of the participants and their guardians. The protocol of the present study was registered a priori and is publicly available on the Open Science Framework Registries platform (DOI: 10.17605/OSF.IO/DU96X).
Data availability
The database for this study is publicly available at: https://figshare.com, under the Doi: 10.6084/m9.figshare.19450583.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
For your support and encouragement for the development of this academic article, we thank the Federal University of Rio Grande do Norte (UFRN), the Physical Activity and Health (AFISA) research base, the Child and Adolescent Maturation Research Group (GEPMAC). The National Council for Scientific Development (CNPQ) and the Higher Education Personnel Improvement Coordination (CAPES).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.smhs.2023.09.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Keiner M., Wirth K., Fuhrmann S., et al. The influence of upper-and lower-body maximum strength on swim block start, turn, and overall swim performance in sprint swimming. J Strength Condit Res. 2021;35(10):2839–2845. doi: 10.1519/JSC.0000000000003229. [DOI] [PubMed] [Google Scholar]
- 2.Marinho D.A., Neiva H.P., Branquinho L., Ferraz R. Determinants of sports performance in young national level swimmers: a correlational study between anthropometric variables, muscle strength, and performance. Sport Mont. 2021;19(3):75–82. doi: 10.26773/smj.211019. [DOI] [Google Scholar]
- 3.Morris K.S., Jenkins D.G., Osborne M.A., et al. The role of the upper and lower limbs in front crawl swimming: the thoughts and practices of expert high-performance swimming coaches. Int J Sports Sci Coach. 2019;14(5):629–638. doi: 10.1177/1747954119866358. [DOI] [Google Scholar]
- 4.Seifert L., Toussaint H.M., Alberty M., Schnitzler C., Chollet D. Arm coordination, power, and swim efficiency in national and regional front crawl swimmers. Hum Mov Sci. 2010;29(3):426–439. doi: 10.1016/j.humov.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Zamparo P., Cortesi M., Gatta G. The energy cost of swimming and its determinants. Eur J Appl Physiol. 2020;120:41–66. doi: 10.1007/s00421-019-04270-y. [DOI] [PubMed] [Google Scholar]
- 6.Gaitanos G.C., Williams C., Boobis L.H., Brooks S. Human muscle metabolism during intermittent maximal exercise. J Appl Physiol. 1993;1985(75):712–719. doi: 10.1152/jappl.1993.75.2.712. [DOI] [PubMed] [Google Scholar]
- 7.Bogdanis G.C., Nevill M.E., Boobis L.H., Lakomy H.K. Contribution of phosphocreatine and aerobic metabolism to energy supply during repeated sprint exercise. J Appl Physiol. 1985;1996(80):876–884. doi: 10.1152/jappl.1996.80.3.876. [DOI] [PubMed] [Google Scholar]
- 8.Cerit M., Dalip M., Yildirim D.S. Genetics and athletic performance. Dissertation. Lokman Hekim Universitesi. 2020 doi: 10.46733/PESH20920065c. [DOI] [Google Scholar]
- 9.Costill D.L., Coyle E.F., Fink W.F., Lesmes G.R., Witzmann F.A. Adaptations in skeletal muscle following strength training. J Appl Physiol Respir Environ Exerc Physiol. 1979;46(1):96–99. doi: 10.1152/jappl.1979.46.1.96. [DOI] [PubMed] [Google Scholar]
- 10.James L.P., Haff G.G., Kelly V.G., Beckman E.M. Towards a determination of the physiological characteristics distinguishing successful mixed martial arts athletes: a systematic review of combat sport literature. Sports Med. 2016;46:1525–1551. doi: 10.1007/s40279-016-0493-1. [DOI] [PubMed] [Google Scholar]
- 11.Slimani M., Nikolaidis P.T. Anthropometric and physiological characteristics of male soccer players according to their competitive level, playing position and age group: a systematic review. J Sports Med Phys Fit. 2017;59(1):141–163. doi: 10.23736/S0022-4707.17.07950-6. [DOI] [PubMed] [Google Scholar]
- 12.Kushmerick M.J. In: Fatigue: Neural and Muscular Mechanisms. Gandevia S.C., Enoka R.M., McComas A.J., Stuart D.G., Thomas C.K., Pierce P.A., editors. Springer Nature; 1995. Bioenergetics and muscle cell types; pp. 175–184. [DOI] [Google Scholar]
- 13.Morouço P., Keskinen K.L., Vilas-Boas J.P., Fernandes R.J. Relationship between tethered forces and the four swimming techniques performance. J Appl Biomech. 2011;27(2):161–169. doi: 10.1123/jab.27.2.161. [DOI] [PubMed] [Google Scholar]
- 14.Mallett A., Bellinger P., Derave W., et al. Muscle fiber typology and its association with start and turn performance in elite swimmers. Int J Sports Physiol Perform. 2021;16(6):834–840. doi: 10.1123/ijspp.2020-0548. [DOI] [PubMed] [Google Scholar]
- 15.Almeida-Neto PF de, de Medeiros Rc da sc, de Matos D.G., et al. Lean mass and biological maturation as predictors of muscle power and strength performance in young athletes. PLoS One. 2021;16(7) doi: 10.1371/journal.pone.0254552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Almeida-Neto P.F., de Assis G.G., de Medeiros J.A., Dantas P.M.S., de Araújo Tinôco Cabral B.G. Muscle power differences between upper and lower limbs in adolescent athletes: an approach of expert researchers. Sport Sci Health. 2023;19:615–623. doi: 10.1007/s11332-022-00928-x. [DOI] [Google Scholar]
- 17.Kumar A., Singh R.K., Apte V.V., Kolekar A. Comparison between seated medicine ball throw test and Wingate test for assessing upper body peak power in elite power sports players. Indian J Physiol Pharmacol. 2021;64(4):286–291. doi: 10.25259/IJPP_36_2021. [DOI] [Google Scholar]
- 18.Özkan A., Köklü Y., Ersöz G. Wingate anaerobic power test. J Appl Soc Sci. 2010;7(1):207–224. https://www.j-humansciences.com/ojs/index.php/IJHS/article/view/942 [Google Scholar]
- 19.Mello J.B., Nagorny G.A.K., Haiachi M.D.C., Gaya A.R., Gaya A.C.A. Projeto Esporte Brasil: physical fitness profile related to sport performance of children and adolescents. Rev. Bras. Cineantropometria Desempenho Hum. 2016;18(6):658–666. doi: 10.5007/1980-0037.2016v18n6p658. [DOI] [Google Scholar]
- 20.Bosco C., Viitasalo J.T., Komi P.V., Luhtanen P. Combined effect of elastic energy and myoelectrical potentiation during stretch-shortening cycle exercise. Acta Physiol Scand. 1982;114(4):557–565. doi: 10.1111/j.1748-1716.1982.tb07024.x. [DOI] [PubMed] [Google Scholar]
- 21.Rakholiya P.A., Gadesha A. A study to correlate the vertical jump test and wingate cycle test as a method to assess anaerobic power in football players. Indian J Public Health Res Dev. 2020;11(7):538–544. [Google Scholar]
- 22.Dos Santos M.A.M., Henrique R.S., Salvina M., et al. The influence of anthropometric variables, body composition, propulsive force and maturation on 50m freestyle swimming performance in junior swimmers: an allometric approach. J Sports Sci. 2021;39(14):1615–1620. doi: 10.1080/02640414.2021.1891685. [DOI] [PubMed] [Google Scholar]
- 23.McKay A.K.A., Stellingwerff T., Smith E.S., et al. Defining training and performance caliber: a participant classification framework. Int J Sports Physiol Perform. 2021;17(2):317–331. doi: 10.1123/ijspp.2021-0451. [DOI] [PubMed] [Google Scholar]
- 24.van Delden J.J.M., van der Graaf R. Revised cioms international ethical guidelines for health-related research involving humans. JAMA. 2017;317(2):135–136. doi: 10.1001/jama.2016.18977. [DOI] [PubMed] [Google Scholar]
- 25.Von Elm E., Altman D.G., Egger M., et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Silva VS da, Vieira M.F.S. International Society for the Advancement of Kinanthropometry (ISAK) global: international accreditation scheme of the competent anthropometrist. Rev Bras Cineantropometria Desempenho Hum. 2020;22 doi: 10.1590/1980-0037.2020v22e70517. [DOI] [Google Scholar]
- 27.Adão Perini T., Lameira de Oliveira G., dos Santos O.J., de Oliveira F.P. Technical error of measurement in anthropometry. Rev Bras Med Esporte. 2005;11:86–90. doi: 10.1590/S1517-86922005000100009. [DOI] [Google Scholar]
- 28.Khadilkar A., Chiplonkar S., Sanwalka N., Khadilkar V., Mandlik R., Ekbote V. A cross-calibration study of GE lunar IDXA and GE lunar DPX pro for body composition measurements in children and adults. J Clin Densitom. 2020;23(1):128–137. doi: 10.1016/j.jocd.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Moore S.A., McKay H.A., Macdonald H., et al. Enhancing a somatic maturity prediction model. Med Sci Sports Exerc. 2015;47(8):1755–1764. doi: 10.1249/MSS.0000000000000588. [DOI] [PubMed] [Google Scholar]
- 30.Crowell B. Light and Matter; 2001. Newtonian Physics. 1st edition. [Google Scholar]
- 31.Cohen J. Quantitative methods in psychology: a power primer. Psychol Bull. 1992;112:1155–1159. doi: 10.1037/0033-2909.112.1.15. [DOI] [Google Scholar]
- 32.Schober P., Boer C., Schwarte L.A. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126(5):1763–1768. doi: 10.1213/ANE.0000000000002864. [DOI] [PubMed] [Google Scholar]
- 33.Dahmane R., Djordjevič S., Šimunič B., Valencic V. Spatial fiber type distribution in normal human muscle: histochemical and tensiomyographical evaluation. J Biomech. 2005;38(12):2451–2459. doi: 10.1016/j.jbiomech.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 34.Van Hall G., Jensen-Urstad M., Rosdahl H., Holmberg H.C., Saltin B., Calbet J.A. Leg and arm lactate and substrate kinetics during exercise. Am J Physiol Endocrinol Metab. 2003;284(1):E193–E205. doi: 10.1152/ajpendo.00273.2002. [DOI] [PubMed] [Google Scholar]
- 35.Calbet J.A., Holmberg H.C., Rosdahl H., van Hall G., Jensen-Urstad M., Saltin B. Why do arms extract less oxygen than legs during exercise. Am J Physiol Regul Integr Comp Physiol. 2005;289(5):R1448–R1458. doi: 10.1152/ajpregu.00824.2004. [DOI] [PubMed] [Google Scholar]
- 36.Helge J.W. Arm and leg substrate utilization and muscle adaptation after prolonged low-intensity training. Acta Physiol. 2010;199(4):519–528. doi: 10.1111/j.1748-1716.2010.02123.x. [DOI] [PubMed] [Google Scholar]
- 37.Ørtenblad N., Nielsen J., Boushel R., Söderlund K., Saltin B., Holmberg H.C. The muscle fiber profiles, mitochondrial content, and enzyme activities of the exceptionally well-trained arm and leg muscles of elite cross-country skiers. Front Physiol. 2018;9:1031. doi: 10.3389/fphys.2018.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lloyd R.S., Oliver J.L., Faigenbaum A.D., et al. Long-term athletic development-part 1: a pathway for all youth. J Strength Condit Res. 2015;29(5):1439–1450. doi: 10.1519/JSC.0000000000000756. [DOI] [PubMed] [Google Scholar]
- 39.Ratel S., Blazevich A.J. Are prepubertal children metabolically comparable to well-trained adult endurance athletes? Sports Med. 2017;47(8):1477–1485. doi: 10.1007/s40279-016-0671-1. [DOI] [PubMed] [Google Scholar]
- 40.Rowland T.W. Human Kinetics; 2005. Children's Exercise Physiology. 1st edition. [Google Scholar]
- 41.Birat A., Bourdier P., Piponnier E., et al. Metabolic and fatigue profiles are comparable between prepubertal children and well-trained adult endurance athletes. Front Physiol. 2018;9:387. doi: 10.3389/fphys.2018.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evangelista T., Kandji M., Lacene M., et al. Other nmds: ep. 355 comprehensive morphometric assessment of skeletal muscle development from birth to 18 years. Neuromuscul Disord. 2021;31:S158. doi: 10.1016/j.nmd.2021.07.380. [DOI] [Google Scholar]
- 43.Kaczor J.J., Ziolkowski W., Popinigis J., Tarnopolsky M.A. Anaerobic and aerobic enzyme activities in human skeletal muscle from children and adults. Pediatr Res. 2005;57(3):331–335. doi: 10.1203/01.PDR.0000150799.77094.DE. [DOI] [PubMed] [Google Scholar]
- 44.Neu C.M., Rauch F., Rittweger J., Manz F., Schoenau E. Influence of puberty on muscle development at the forearm. Am J Physiol Endocrinol Metab. 2002;283(1):E103–E107. doi: 10.1152/ajpendo.00445.2001. [DOI] [PubMed] [Google Scholar]
- 45.Richmond E., Rogol A.D. Endocrine responses to exercise in the developing child and adolescent. Sports Endoc. 2016;47:58–67. doi: 10.1159/000445157. [DOI] [PubMed] [Google Scholar]
- 46.Almeida-Neto PF de, de Matos D.G., Pinto V.C.M., et al. Can the neuromuscular performance of young athletes be influenced by hormone levels and different stages of puberty? Int J Environ Res Publ Health. 2020;17(16):5637. doi: 10.3390/ijerph17165637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sizonenko P.C. Endocrinology in preadolescents and adolescents: hormonal changes during normal puberty. Am J Dis Child. 1978;132(7):704–712. doi: 10.1001/archpedi.1978.02120320064015. [DOI] [PubMed] [Google Scholar]
- 48.Cavarzere P., Gaudino R., Sandri M., et al. Growth hormone retesting during puberty: a cohort study. Eur J Endocrinol. 2020;182(6):559–567. doi: 10.1530/EJE-19-0646. [DOI] [PubMed] [Google Scholar]
- 49.Sundberg C.W., Fitts R.H. Bioenergetic basis of skeletal muscle fatigue. Curr Opin Physiol. 2019;10:118–127. doi: 10.1016/j.cophys.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zamparo P., Bonifazi M. In: Nutrition and Enhanced Sports Performance. Bagchi D., Nair S., Sen K.C., editors. Elsevier; 2019. Bioenergetics of cyclic sport activities in water: swimming, rowing, and kayaking; pp. 141–149. 2nd edition. [DOI] [Google Scholar]
- 51.Schulkin J. Columbia University Press; 2017. Throwing, Swimming, and Rowing; pp. 115–136. 1st edition. [DOI] [Google Scholar]
- 52.Nikšić E., Beganović E., Joksimović M., et al. The impact of strength and coordination on the success of performance of the freestyle swimming. Eur J Sport Sci. 2019;5(11):10–22. doi: 10.5281/zenodo.33640. [DOI] [Google Scholar]
- 53.Faigenbaum A.D., French D.N., Lloyd R.S., et al. In: Strength and Conditioning for Young Athletes. Lloyd R.S., Oliver J.L., editors. Routledge; 2019. Strength and power training for young athletes; pp. 131–154. 2nd edition. [DOI] [Google Scholar]
- 54.Morais J.E., Silva A.J., Garrido N.D., Marinho D.A., Barbosa T.M. The transfer of strength and power into the stroke biomechanics of young swimmers over a 34-week period. Eur J Sport Sci. 2018;18(6):787–795. doi: 10.1080/17461391.2018.1453869. [DOI] [PubMed] [Google Scholar]
- 55.Morais J.E., Silva A.J., Marinho D.A., Lopes V.P., Barbosa T.M. Determinant factors of long-term performance development in young swimmers. Int J Sports Physiol Perform. 2017;12(2):198–205. doi: 10.1123/ijspp.2015-0420. [DOI] [PubMed] [Google Scholar]
- 56.Kováčová N., Broďáni J. Swimming performance to 25 meters backstroke depends on selected factors of explosive strength of lower limbs. Acta fac educ phys Univ Comene. 2019;59(2):203–213. doi: 10.2478/afepuc-2019-0018. [DOI] [Google Scholar]
- 57.Gourgoulis V., Boli A., Aggeloussis N., et al. The effect of leg kick on sprint front crawl swimming. J Sports Sci. 2014;32(3):278–289. doi: 10.1080/02640414.2013.823224. [DOI] [PubMed] [Google Scholar]
- 58.Almeida-Neto PF de, Matos DG de, Baxter-Jones A.D.G., et al. The effectiveness of biological maturation and lean mass in relation to muscle strength performance in elite young athletes. Sustainability. 2020;12(17):6696. doi: 10.3390/su12176696. [DOI] [Google Scholar]
- 59.Hawley J.A., Williams M.M. Relationship between upper body anaerobic power and freestyle swimming performance. Int J Sports Med. 1991;12(1):1–5. doi: 10.1055/s-2007-1024645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The database for this study is publicly available at: https://figshare.com, under the Doi: 10.6084/m9.figshare.19450583.