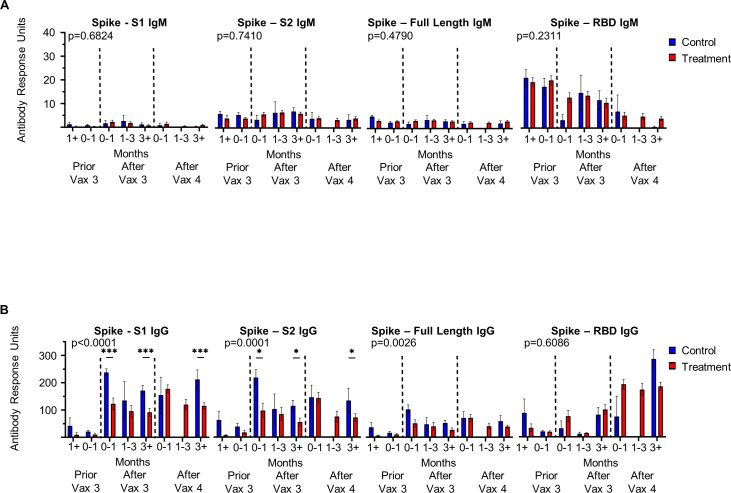
Figure 3.
Antineoplastic and immunomodulating agents impair immunoglobulin class switching to SARS-CoV-2 Spike subunits. (A) IgM and (B) IgG antibody response to respective SARS-CoV-2 epitopes at time points following third (Vax 3) and fourth (Vax 4) COVID-19 immunization in patients receiving antineoplastic and immunomodulating agents (treatment) and immunocompetent patients (control) (Immunocompetent: greater than 1 month prior to Vax 3 n=4, 0–1 month prior to Vax 3 n=9, 0–1 month following Vax 3 n=9, 1–3 months following Vax 3 n=3, greater than 3 months following Vax 3 n=17, 0–1 month following Vax 4 n=2, 1–3 months following Vax 4 n=0, greater than 3 months following Vax 4 n=4; Immunosuppressed: greater than 1 month prior to Vax 3 n=5, 0–1 month prior to Vax 3 n=18, 0–1 month following Vax 3 n=20, 1–3 months following Vax 3 n=19, greater than 3 months following Vax 3 n=36, 0–1 month following Vax 4 n=28, 1–3 months following Vax 4 n=17, greater than 3 months following Vax 4 n=27). Dashed lines indicate Vax three and Vax 4. Antibody response reported as antibody response units. When significance observed by ANOVA, significant pairwise comparisons indicated by *p<0.05, ***p<0.001 by Tukey’s multiple comparison. ANOVA, analysis of variance; RBD, receptor binding domain.