Fig. 3.
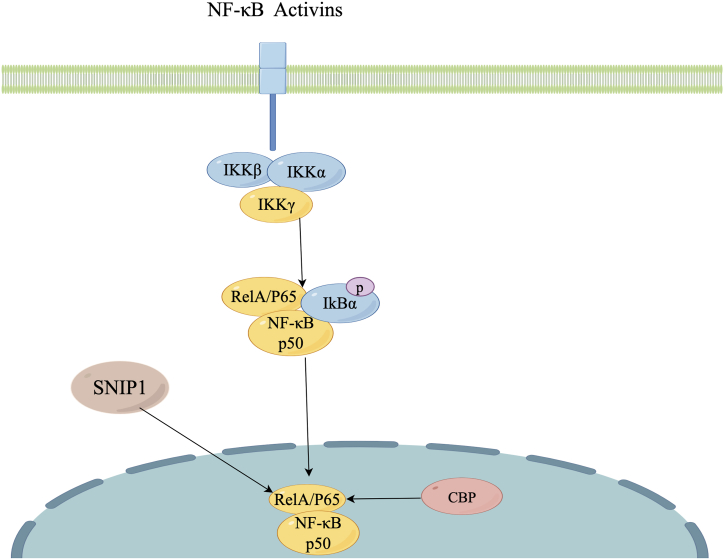
Within the NF-κB signaling pathway, SNIP1 demonstrates the capacity to interact with RelA/P65.
Inflammatory mediators, including proinflammatory cytokines, lipopolysaccharide (LPS), growth factors, and antigen receptors, initiate the activation of an IKK complex that comprises IKKβ, IKKα, and IKKγ. This complex then phosphorylates IκB proteins, which binding to NF‐κB, preventing their nuclear translocation and binding to DNA. The phosphorylation of IκB causes its own ubiquitination and subsequent degradation through the proteasome, leading to the liberation of the NF-κB/Rel complex. The active NF-κB/Rel complex undergoes further activation through posttranslational modifications such as phosphorylation, acetylation, and glycosylation. Subsequently, it translocates into the nucleus and, in conjunction with CBP or other transcriptional factors, exerts its transcriptional regulatory functions. Furthermore, SNIP1 engages in competition with CBP for the binding to RelA/p65, resulting in the inhibition of NF-κB activity.