Abstract
Background
Stress is a potent activator of the hypothalamic-pituitary-adrenal (HPA) axis, initiating the release of glucocorticoid hormones, such as cortisol. Alcohol consumption can lead to HPA axis dysfunction, including altered cortisol levels. Until recently, research has only been able to examine peripheral cortisol associated with alcohol use disorder (AUD) in humans. We used positron emission tomography (PET) brain imaging with the radiotracer [18F]AS2471907 to measure 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), a cortisol-regenerating enzyme, in people with AUD compared to healthy controls.
Methods
We imaged 9 individuals with moderate to severe AUD (5 men, 4 women; mean age = 38 years) and 12 healthy controls (8 men, 4 women; mean age = 29 years). Participants received 93.5 ± 15.6 MBq of the 11β-HSD1 inhibitor radiotracer [18F]AS2471907 as a bolus injection and were imaged for 150–180 min on the High-Resolution Research Tomograph. 11β-HSD1 availability was quantified by [18F]AS2471907 volume of distribution (VT; mL/cm3). A priori regions of interest included amygdala, anterior cingulate cortex (ACC), hippocampus, ventromedial PFC (vmPFC) and caudate.
Results
Individuals with AUD consumed 52.4 drinks/week with 5.8 drinking days/week. Healthy controls consumed 2.8 drinks/week with 1.3 drinking days/week. Preliminary findings suggest that [18F]AS2471907 VT was higher in amygdala, ACC, hippocampus, vmPFC, and caudate of those with AUD compared to healthy controls (p < 0.05). In AUD, vmPFC [18F]AS2471907 VT was associated with drinks per week (r = 0.81, p = 0.01) and quantity per drinking episode (r = 0.75, p = 0.02).
Conclusions
This is the first in vivo examination of 11β-HSD1 availability in individuals with AUD. Our data suggest higher brain availability of the cortisol-regenerating enzyme 11β-HSD1 in people with AUD (vs. controls), and that higher vmPFC 11β-HSD1 availability is related to greater alcohol consumption. Thus, in addition to the literature suggesting that people with AUD have elevated peripheral cortisol, our findings suggest there may also be heightened central HPA activity. These findings set the foundation for future hypotheses on mechanisms related to HPA axis function in this population.
Keywords: Alcohol, Alcohol use disorder, Cortisol, 11β-HSD1, Stress, PET imaging
Highlights
-
•
Alcohol consumption can lead to HPA axis dysfunction, including altered cortisol levels.
-
•
We have only been able to examine peripheral cortisol associated with AUD in humans thus far.
-
•
We used PET imaging and the novel radiotracer [18F]AS2471907 to measure 11β-HSD1, a cortisol-regenerating enzyme, in AUD.
-
•
[18F]AS2471907 VT was higher in amygdala, ACC, hippocampus, vmPFC, and caudate in AUD vs. HC.
-
•
Our findings suggest that people with AUD may also have heightened central HPA activity.
1. Introduction
One of the principal drivers associated with the maintenance of and relapse to alcohol use is stress (Koob, 2009; Peltier et al., 2019). Stress is a potent activator of the hypothalamic pituitary adrenal (HPA) axis, and, through a downstream cascade, activates the release of glucocorticoid hormones (i.e., cortisol) from the adrenal glands (Koob and Heinrichs, 1999). Numerous studies have shown that acute and chronic alcohol use dysregulate the HPA axis system, and this dysregulation influences the frequency and escalation of alcohol consumption (Blaine et al., 2016; Peltier et al., 2019). Specifically, chronic alcohol use is associated with elevated tonic levels of peripheral cortisol, and this tonic HPA hyperreactivity may blunt the ability of the HPA axis to respond to stress in individuals with alcohol use disorder (AUD) versus healthy individuals (Dunne and Ivers, 2023). However, results from this aspect of research are mixed, with studies finding both lower and higher peripheral cortisol responses to stress (Dunne and Ivers, 2023), suggesting a need to better characterize HPA dysregulation in this population.
Alterations in HPA axis reactivity may be driven by the enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1). 11β-HSD1 is a reductase that catalyzes the conversion of inactive cortisone to cortisol (Chapman et al., 2013). As previously noted by our group and others, 11β-HSD1 is estimated to contribute to at least 50% of cortisol in the brain (Bhatt et al., 2021; Yau et al., 2001). [18F]AS2471097 is a novel radiotracer with specific binding to 11β-HSD1 (Baum et al., 2019), allowing for in vivo examination of the enzyme in living humans (Bhatt et al., 2019; 2021).
Preclinical work indicates chronic alcohol exposure increases both brain and peripheral corticosterone, the rodent equivalent of cortisol, with some studies suggesting greater increases in the brain (Becker 2017; Edwards et al., 2015; Little et al., 2008). Corticosterone facilitates greater alcohol consumption in rodents when given orally, and this is also true when only the brain is exposed to corticosterone (Fahlke et al., 1994; Fahlke et al., 1996; Fahlke and Erikson, 2000). However, brain cortisol regulation in people with AUD is not well understood. With the current investigation, our aim was to measure 11β-HSD1 levels with in vivo positron emission tomography (PET) brain imaging using the 11β-HSD1-specfic radioligand [18F]AS2471097 in people with AUD vs. controls. Given the preclinical literature, we hypothesized that individuals with AUD would have higher levels of 11β-HSD1 in a prefrontal-limbic circuit, composed of key regions associated with AUD (Koob, 2009), compared to controls.
2. Material and methods
2.1. Participant characteristics
Nine individuals with AUD (5 men, 4 women) and 12 healthy control individuals (8 men, 4 women) participated in the study. Individuals with AUD were not seeking treatment for their drinking. Participants were between 24 and 50 years old. All participants provided written informed consent. Procedures were in accordance with the ethical standards of the Yale School of Medicine Human Investigation Committee and the Yale New Haven Hospital Radiation Safety Committee. The Structured Clinical Interview (SCID) for Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM-5) was used to confirm diagnostic inclusion and exclusion criteria (First et al., 2016). We recorded alcohol use over the prior 30 days with the Timeline-Follow Back Interview (TLFB) (Sobell and Sobell, 1992) and further assessed severity of AUD with the Alcohol Dependence Scale (Skinner, 1984). Participants underwent medical screening, including a physical exam, an electrocardiogram (EKG), basic blood chemistries, urine drug toxicology screen, and a blood pregnancy test for women.
Individuals with AUD had a current diagnosis (past 6-months) with reported drinking of >14 drinks per week and >4 drinks per day at least twice per week for men and >7 drinks per week and >3 drinks per day at least twice per week in women. Participants met this drinking criteria during a consecutive 30-day period prior to intake (i.e., in person eligibility intake). Participants were excluded if they met DSM-5 criteria for other primary psychiatric and substance use disorders (excluding post-traumatic stress disorder [PTSD], major depressive disorder [MDD], anxiety, and nicotine dependence), used illicit drugs, were seeking treatment for their alcohol use, had current suicidal or homicidal ideation, were pregnant or nursing, or were likely to exhibit clinically significant alcohol withdrawal (Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised [CIWA-R] ≥ 8) (Sullivan et al., 1989). Individuals with AUD and healthy controls were required to be overnight abstinent from alcohol.
2.2. Imaging acquisition
Participants were administered an intravenous bolus injection of 92.4 ± 6.3 MBq for the AUD group and 94.3 ± 4 MBq for the healthy control group of [18F]AS2471097, synthesized as previously described (Baum et al., 2019). Participants were imaged for 150–180 min after injection on a High-Resolution Research Tomograph (HRRT; Siemens, Medical Solutions) with a 30-min break starting at 90 or 120 min. All participants were scanned starting between 11:00 a.m. and 2:00 p.m., to reduce temporal variability in well-established diurnal cortisol rhythms (Stone et al., 2001). A structural image for anatomical co-registration was acquired with a high-resolution T1-weighted MRI with a sagittal gradient-echo (MPRAGE) sequence (Siemens 3.0T Prisma Fit; 176 sagittal slices, thickness = 1 mm, TR = 2530 ms, TE = 2.26 ms, flip angle = 7°, FoV = 256 mm, matrix size = 256 × 256).
2.3. Arterial input function measurement
An arterial catheter was placed in the radial artery contralateral to the injection site for [18F]AS2471907, for arterial sampling throughout the duration of the scan. Radioactivity was measured in manual samples collected rapidly from 0 to 2.5 min, then at 2.75, 3, 5, 10, 20, and 30 min, and every 15 min thereafter until 120 min or 150 min, then every 30 min for scans longer than 150 min. Fraction of unmetabolized [18F]AS2471907 was measured using high-performance liquid chromatography (HPLC) as previously described (Hilton et al., 2000) in plasma samples taken at 0, 10, 30, 60, 90, 120, and 150 min, or every 60 min after the 120-min time point for scans longer than 150 min. A metabolite-corrected arterial input function was calculated as the product of the plasma radioactivity and unmetabolized [18F]AS2471907 fraction, with radioactivity measured with a cross-calibrated well counter (1480 Wizard, Perkin-Elmer).
2.4. PET imaging analysis
PET data were collected in list-mode and reconstructed using MOLAR (Carson et al., 2003) with correction for attenuation, scatter, randoms, deadtime, and subject motion as recorded by Vicra (Polaris Vicra Optical Tracking System, NDI Systems). A 7-min transmission scan was performed prior to radiotracer injection and immediately following completion of full PET data acquisition, with the latter transmission scan allowing attenuation correction for potential shifts in participant position in the scanner following the 30-min break. A 0 to 10-min summed PET image was linearly co-registered to each individual's MRI. Nonlinear transformation from Montreal Neurological Institute (MNI) atlas space to individual MRI space and an anatomic automatic labeling (AAL) template were used to generate time activity curves in amygdala, caudate, anterior cingulate cortex (ACC), hippocampus, and ventromedial prefrontal cortex (vmPFC) regions of interest (ROIs), as these regions are heavily implicated in stress pathophysiology and addiction (George et al., 2001; Goldstein and Volkow, 2002; Koob, 2009; Roberto et al., 2010). Based on previous characterization of optimal modeling methods (Bhatt et al., 2019), multilinear analysis (MA1) with t* = 30 min was used to estimate regional [18F]AS2471907 volume of distribution or VT, defined as the ratio of radioactivity in tissue to plasma at equilibrium (Innis et al., 2007). Voxel-wise parametric images were generated using 1T compartment model and smoothing with a 7 mm Gaussian kernel, as previously described (Bhatt et al., 2019).
2.5. Statistical analysis
Demographic characteristics and injection parameters were compared between the AUD and healthy control groups using Student's t tests or χ2 tests, where appropriate. The main effect of group on prefrontal-limbic 11β-HSD1 availability was examined using multivariate general linear modeling (GLM), with [18F]AS2471907 VT as the dependent variable, group as a between-subject factor, and ROI as a within-subject factor for the following a priori ROIs: amygdala, caudate, ACC, hippocampus, and vmPFC. Multivariate GLM limits experiment wise error of multiple comparisons at p = 0.05. Pearson correlation coefficients were calculated for exploratory associations between 30-day averages prior to intake of drinking days per week, drinks per week, and quantity per drinking episode and severity of alcohol dependence with [18F]AS2471907 VT values in all a priori ROIs. For all statistical analyses, a p value < 0.05 was considered the threshold for statistical significance. Age was included as a co-variate in the multivariate GLM analysis because 11β-HSD1 has been found to be associated with age and age-related cognitive decline (Baum et al., 2019; Sandeep et al., 2004).
3. Results
3.1. Participants
Nine individuals with AUD and 12 healthy controls participated in the study. Groups were matched on demographic characteristics including sex, BMI, and radiotracer injection parameters (ps > 0.05). Age, smoking status, and ethnicity were significantly different between groups (p < 0.05), with a greater number of individuals in the AUD group being older, individuals who smoke, and self-identified as Black (see Table 1). Drinking characteristics are outlined in Table 1. As expected, individuals in the AUD group consumed significantly more alcohol compared to healthy controls.
Table 1.
Participant characteristics.
| AUD (n = 9) | HC (n = 12) | p | |
|---|---|---|---|
| Age, years | 37.9 ± 2.9 | 29.4 ± 1.2 | 0.01 |
| Body mass index | 26.6 ± 1.4 | 26.8 ± 1.5 | 0.95 |
| Sex (n) | 0.60 | ||
| Female | 4 | 4 | |
| Male | 5 | 8 | |
| Race/Ethnicity (n) | 0.01 | ||
| White | 1 | 5 | |
| Black | 8 | 2 | |
| Hispanic | 0 | 3 | |
| Other | 0 | 2 | |
| Individuals who smoke (%) | 87.5 | 8.3 | .001 |
| Timeline Follow-Back | |||
| Drinking days/week | 5.8 ± 0.4 | 1.3 ± 0.6 | <0.001 |
| Drinks/week | 52.4 ± 9.8 | 2.8 ± 1.4 | <0.001 |
| Drinks/episode | 9.1 ± 1.3 | 1.4 ± 0.4 | <0.001 |
| Injection parameters | |||
| Injected dose (MBq) | 92.4 ± 6.3 | 94.3 ± 4.1 | 0.45 |
| Injected mass (μg) | 0.5 ± 0.1 | 0.7 ± 0.2 | 0.40 |
HC: healthy controls, AUD: alcohol use disorder. Values are mean ± SE.
3.2. Group differences in 11β-HSD1 in the prefrontal-limbic circuit
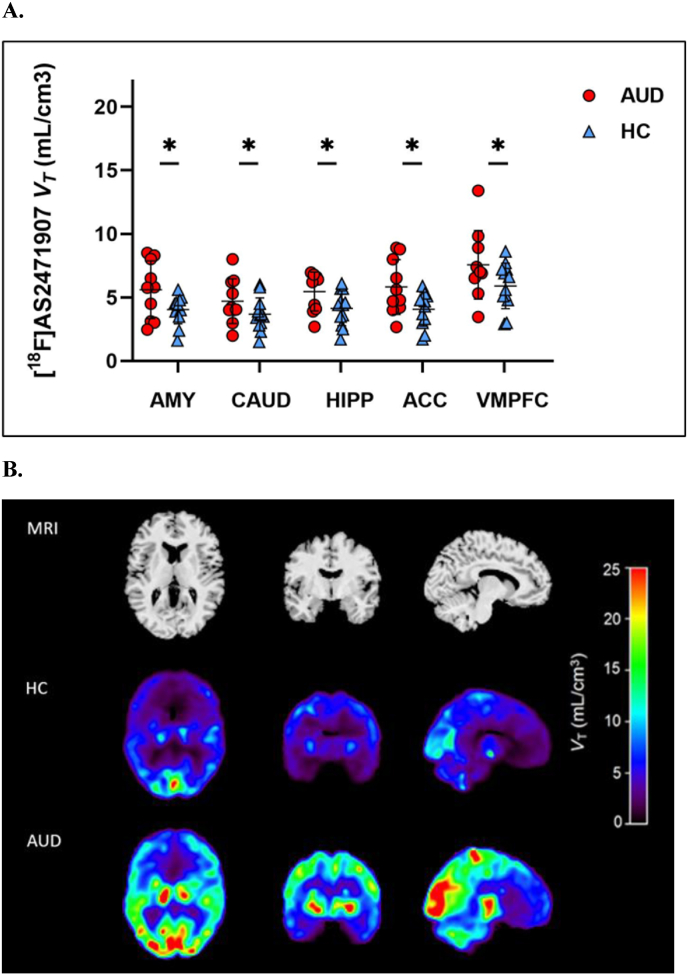
There was a significant overall main effect of group in the prefrontal-limbic circuit (F(2,20) = 5.60, p = 0.01; Fig. 1A), with 41.7% higher overall 11β-HSD1 in the AUD group compared to the healthy control group. Group comparisons in each ROI revealed a main effect of group for amygdala (F(2, 20) = 5.14, p = 0.02), caudate (F(2,20) = 3.83, p = 0.04), ACC (F(2,20) = 5.90, p = 0.01), hippocampus (F(2,20) = 3.82, p = 0.04), and vmPFC (F(2,20) = 4.99, p = 0.02). Mean [18F]AS2471907 VT values were higher in the AUD group by 47.0% in amygdala, 36.5% in caudate, 51.6% in ACC, 39.4% in hippocampus, and 35.9% in vmPFC after adjusting for age as a covariate. Shown in Fig. 1B are representative parametric images of [18F]AS2471907 VT from an individual with AUD and an age- and sex-matched control.
Fig. 1.
(A) Regional VT values in individuals with AUD (n = 9; red circles) and healthy controls (n = 12; blue triangles). [18F]AS2471907 VT is significantly higher in prefrontal-limbic brain regions in people with AUD vs. controls (*all ps < 0.05). AMY, amygdala; CAUD, caudate; HIPP, hippocampus; ACC, anterior cingulate cortex; VMPFC, ventromedial prefrontal cortex. (B) A representative participant's MRI (top panel), and co-registered parametric [18F]AS2471907 VT image of a healthy control (middle panel) and an age- and sex-matched individual with AUD (bottom panel). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Relationships between 11β-HSD1 and drinking characteristics in AUD
11β-HSD1 availability in vmPFC was significantly associated with drinks per week (r = 0.81, p = 0.01) and quantity per drinking episode (r = 0.75, p = 0.02) in the 30-days prior to intake. Alcohol dependence severity was significantly associated with [18F]AS2471907 VT in the ACC (r = 0.77, p = 0.03) and vmPFC (r = 0.76, p = 0.03).
4. Discussion
This is the first in vivo examination of 11β-HSD1, a cortisol-regenerating enzyme, in individuals with AUD. Overall, individuals with AUD demonstrated higher 11β-HSD1 availability compared to healthy individuals in all prefrontal-limbic regions, including amygdala, caudate, ACC, hippocampus, and vmPFC. These regions are heavily implicated in stress pathophysiology and addiction, or the ‘dark side of addiction’ (Koob, 2009). We also examined relationships between 11β-HSD1 availability and drinking in the 30 days prior to intake in our sample of individuals with AUD. We found that higher 11β-HSD1 availability in vmPFC was associated with drinks per week, quantity of drinks per drinking episode, and alcohol dependence severity, suggesting consistent relationships between drinking behavior and 11β-HSD1 availability in vmPFC. Thus, in addition to the literature suggesting that people with AUD have elevated peripheral cortisol, our findings suggest there may also be heightened central HPA activity.
Our findings indicating elevated 11β-HSD1 availability in participants with AUD are consistent with preclinical literature. Chronic alcohol exposure induced prolonged increases in brain glucocorticoid levels in rodents that persisted after plasma corticosterone returned to baseline (Little et al., 2008). Relatedly, in rodents with chronic high ethanol exposure history, glucocorticoid receptor expression (mRNA levels) was elevated following chronic alcohol exposure in the central amygdala; an effect not found in rodents with acute ethanol exposure (Vendruscolo et al., 2012). Preclinical work also suggests that acute abstinence from binge-like alcohol consumption is related to corticotropin-releasing factor (CRF), a mediator of the HPA axis response, neuron dysfunction in the medial PFC, and dysfunction between the medial PFC and central amygdala may be critical for alcohol-motivated behavior (George et al., 2012). Given the importance of brain corticosterone levels on alcohol intake in preclinical findings and the role that stress plays in the ‘dark side of addiction’, it will be important to determine if these relationships translate to humans.
We did not exclude for PTSD, MDD, anxiety, and nicotine dependence as these conditions are highly comorbid with AUD (Peltier et al., 2019). Approximately 80% of individuals with AUD smoke cigarettes, and in the U.S., individuals with AUD are 3 times more likely to smoke than the general population (Castillo-Carniglia et al., 2019). In this study, 87.5% of individuals with AUD smoked cigarettes. In addition, the estimated prevalence of comorbid AUD and MDD or anxiety disorders is 20–40% across countries, and 55% of individuals with AUD also meet criteria for PTSD (Castillo-Carniglia et al., 2019). Future work should address the intersection of stress and nicotine and other psychiatric comorbidities in AUD, especially using novel radiotracers such as [18F]AS2471907. In fact, work by our group has recently demonstrated that individuals with PTSD have higher brain cortisol-producing 11β-HSD1 in prefrontal-limbic regions compared to trauma controls (Bhatt et al., 2021).
Limitations of this study are important to address. Briefly, [18F]AS2471907 VT gives a measure of the number of binding sites on 11β-HSD1 available for radiotracer binding, but further studies are needed to fully extrapolate 11β-HSD1 availability to enzyme activity. Second, there was an age difference in participants with AUD compared to controls. Therefore, results were covaried for age as 11β-HSD1 has been shown to change with age (Baum et al., 2019; Sandeep et al., 2004). Findings were not altered with the addition of age as a covariate. Third, this study was not powered to analyze data by sex. This will be important in future investigations as most previous studies on peripheral cortisol in AUD have been conducted in men (Dunne and Ivers, 2023), and research suggests that women may be more likely to drink for stress-related reasons (Peltier et al., 2019). Fourth, we did not systemically collect peripheral cortisol levels. It will be important for future work to directly examine the correspondence between brain and peripheral cortisol in the AUD population, especially given the discrepancies in the literature on peripheral cortisol levels and alcohol consumption. Finally, our AUD group was comprised of 88% of individuals self-identifying as Black, whereas the healthy control sample was comprised of more diverse racial and ethnic backgrounds. This should be considered when interpreting our preliminary results of higher 11β-HSD1 availability in AUD compared to controls. Research has demonstrated race-related differences in chronic stress exposure. Black individuals are more likely to be exposed to a greater number of ongoing stressors compared to white individuals (Brown et al., 2020), and that increases in stress are predictive of increases in depression in Black compared to white individuals. Studies examining diurnal peripheral cortisol rhythms found that Black individuals have flatter cortisol slopes compared to white individuals, although results are mixed (Berger and Sarnyai, 2015). This may suggest that racial differences may be found regarding 11β-HSD1 availability. Future work should explore this possibility.
5. Future research
These preliminary findings are ‘first-in-kind’, and future studies will further expand knowledge regarding central cortisol dysregulation in AUD. AUD and glucocorticoid levels are associated with neurocognitive dysfunction (Baum et al., 2019), and it will be important for future work to examine cognition and 11β-HSD1 levels in relation to AUD. It will also be important to examine adversity, stressors, and race-related stressors. We did not have a measure that addressed racism or race-related stressors, specifically, in this study. This should be a factor in future work, especially in samples comprised primarily of individuals identifying as persons of color. Future studies may benefit from pre-testing for heterogeneity of the targeted stress response with peripheral markers which could direct future investigations of stress responders vs. non-responders. Finally, in an AUD sample, it will be important to examine change across time and how this might predict relapse or return to heavy alcohol use.
6. Conclusions
Overall, preliminary findings indicate that individuals with AUD have higher 11β-HSD1 availability than healthy individuals, in brain regions specific to the ‘dark side of addiction’ (Koob et al., 2009). It is plausible then that higher 11β-HSD1 availability in the brain may drive the blunted response to stress in individuals with AUD, leading to increased vulnerability to excessive use. Given the complex nature of the relationship between alcohol use and HPA axis dysfunction, we hope these findings set the foundation for future hypotheses on mechanisms related to the HPA axis in AUD.
Funding
This work was supported by the National Institutes of Health [K01AA025670, F30MH116607, U54AA027989, R56MH116941].
CRediT authorship contribution statement
Terril L. Verplaetse: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. Ansel T. Hillmer: Formal analysis, Methodology, Software, Supervision, Writing – review & editing, Data curation. Shivani Bhatt: Formal analysis, Funding acquisition, Investigation, Writing – review & editing, Data curation. Aleksandra Rusowicz: Investigation, Writing – review & editing. Songye Li: Resources, Software, Validation, Writing – review & editing. Nabeel Nabulsi: Resources, Software, Validation, Writing – review & editing. David Matuskey: Investigation, Supervision, Writing – review & editing. Yiyun Huang: Funding acquisition, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – review & editing. Sherry A. McKee: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing – review & editing. Kelly P. Cosgrove: Conceptualization, Formal analysis, Funding acquisition, Methodology, Resources, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Rita Valentino
References
- Baum E., et al. A novel 18F-labeled radioligand for positron emission tomography imaging of 11β-hydroxysteroid dehydrogenase (11β-HSD1): synthesis and preliminary evaluation in nonhuman primates. ACS Chem. Neurosci. 2019;10(5):2450–2458. doi: 10.1021/acschemneuro.8b00715. [DOI] [PubMed] [Google Scholar]
- Becker Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology. 2017;122:115–126. doi: 10.1016/j.neuropharm.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., Sarnyai Z. “More than skin deep”: stress neurobiology and mental health consequences of racial discrimination. 2015;18(1):1–10. doi: 10.3109/10253890.2014.989204. [DOI] [PubMed] [Google Scholar]
- Bhatt S., et al. First in-human PET study and kinetic evaluation of [18F]AS2471907 for imaging 11β-hydroxysteroid dehydrogenase type 1. J. Cerebr. Blood Flow Metabol. 2019;40(4):695–704. doi: 10.1177/0271678X19838633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S., et al. Imaging brain cortisol regulation in PTSD with a target for 11β-hydroxysteroid dehydrogenase type 1. J. Clin. Investig. 2021;131(20) doi: 10.1172/JCI150452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine S.K., Milivojevic V., Fox H., Sinha R. Alcohol effects on stress pathways impact on craving and relapse risk. Can. J. Psychiatr. 2016;61(3):145–153. doi: 10.1177/0706743716632512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L.L., Uchechi M.A., Ailshire J.A. Disentangling the stress process: race/ethnic differences in the exposure and appraisal of chronic stressors among older adults. J. Geol. B. 2020;75(3):650–660. doi: 10.1093/geronb/gby072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson R.E., et al. Paper Presented at: 2003 IEEE Nuclear Science Symposium; October 19–25. Portland, Oregon; USA: 2003. Design of a motion-compensation OSEM list-mode algorithm for resolution- recovery reconstruction for the HRRT. [DOI] [Google Scholar]
- Castillo-Carniglia A., Keyes K.M., Hasin D.S., Cerda M. Psychiatric comorbidities in alcohol use disorder. Lancet Psychiatr. 2019;6(12):1068–1080. doi: 10.1016/S2215-0366(19)30222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K., et al. 11β-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol. Rev. 2013;93(3):1139–1206. doi: 10.1152/physrev.00020.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne N., Ivers J.H. HPA axis function in alcohol use disorder: a systemic review and meta-analysis. Addict. Neurosci. 2023;8 [Google Scholar]
- Edwards S., Little H.J., Richardson H.N., Vendruscolo L.F. vol. 49. 2015. pp. 811–816. (Divergent Regulation of Distinct Glucocorticoid Systems in Alcohol Dependence). 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C., Håard E., Hansen S. Facilitation of ethanol consumption by intracerebroventricular infusions of corticosterone. Psychopharmacology. 1996;127:133–139. doi: 10.1007/BF02805986. [DOI] [PubMed] [Google Scholar]
- Fahlke C., Engel J.A., Eriksson C.P.J., Hard E., Soderpalm B. Involvement of corticosterone in the modulation of ethanol consumption in the rat. Alcohol. 1994;3:195–202. doi: 10.1016/0741-8329(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Fahlke C., Erikson C.J. Effect of adrenalectomy and exposure to corticosterone on alcohol intake in alcohol preferring and alcohol avoiding rat lines. Alcohol. 2000;35:139–144. doi: 10.1093/alcalc/35.2.139. [DOI] [PubMed] [Google Scholar]
- First M.B., Williams J.B.W., Karg R.S., Spitzer R.L. Clinical version; 2016. User's Guide for the SCID-5-CV Structured Clinical Interview for DSM-5® Disorders. [Google Scholar]
- George M.S., Anton R.F., Bloomer C., Teneback C., Drobes D.J., Lorberbaum J.P., et al. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch. Gen. Psychiatr. 2001;58(4):345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- George O., Sanders C., Freiling J., Koob G.F. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc. Natl. Acad. Sci. USA. 2012;109(44):18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R.Z., Volkow N.D. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatr. 2002 doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton J., et al. Column-switching HPLC for the analysis of plasma in PET imaging studies. Nucl. Med. Biol. 2000;27(6):627–630. doi: 10.1016/s0969-8051(00)00125-6. [DOI] [PubMed] [Google Scholar]
- Innis R.B., et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J. Cerebr. Blood Flow Metabol. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Koob G.F. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., Heinrichs S.C. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848(1):141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Little H.J., Croft A.P., O'Callaghan M.J., Brooks S.P., Wang G., Shaw S.G. Selective increases in regional brain glucocorticoid: a novel effect of chronic alcohol. Neuroscience. 2008;156(4):1017–1027. doi: 10.1016/j.neuroscience.2008.08.029. [DOI] [PubMed] [Google Scholar]
- Peltier M.R., Verplaetse T.L., Mineur Y.S., Petrakis I.L., Cosgrove K.P., Picciotto M.R., McKee S.A. Sex differences in stress-related alcohol use. Neurobiol. Stress. 2019;10 doi: 10.1016/j.ynstr.2019.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M., Cruz M.T., Gilpin N.W., Sabino V., Schweitzer P., Bajo M., et al. Corticotropin releasing factor–induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol. Psychiatr. 2010;67(9):831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandeep T.C., Yau J.L., MacLullich A.M., Noble J., Deary I.J., Walker B.R., Seckl J.R. 11β-hydroxysteroiddehydrogenase inhibition improves cognitive function in healthyelderly men and type 2 diabetics. Proc. Natl. Acad. Sci. USA. 2004;101:6734–6739. doi: 10.1073/pnas.0306996101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner H. Addiction Research Foundation; Toronto: 1984. Alcohol Dependence Scale. Users Guide. [Google Scholar]
- Sobell L.C., Sobell M.B. Springer; 1992. Timeline Follow-Back. Measuring Alcohol Consumption; pp. 41–72. [Google Scholar]
- Stone A.A., et al. Individual differences in the diurnal cycle of salivary free cortisol: a replication of flattened cycles for some individuals. Psychoneuroendocrinology. 2001;26(3):295–306. doi: 10.1016/s0306-4530(00)00057-3. [DOI] [PubMed] [Google Scholar]
- Sullivan J.T., Sykora K., Schneiderman J., Naranjo C.A., Sellers E.M. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA‐Ar) Br. J. Addiction. 1989;84(11):1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Vendruscolo L.F., Barbier E., Schlosburg J.E., Misra K.K., et al. Corticosteroid-dependent plasticity mediate compulsive alcohol drinking in rats. J. Neurosci. 2012;32 doi: 10.1523/JNEUROSCI.0069-12.2012. 7653-7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau J.L., et al. Lack of tissue glucocorticoid reactivation in 11beta -hydroxysteroid dehydrogenase type 1 knockout mice ameliorates age-related learning impairments. Proc. Natl. Acad. Sci. U.S.A. 2001;98(8):4716–4721. doi: 10.1073/pnas.071562698. [DOI] [PMC free article] [PubMed] [Google Scholar]