Abstract
Patient group It is estimated that 15–30% of geriatric cats will develop chronic kidney disease (CKD), and that 30–65% of these cats will develop anemia as their renal disease worsens. Anemia of renal disease is multifactorial in its pathogenesis, but the main cause is reduced production of erythropoietin, a renal hormone that controls the bone marrow's production of red blood cells, as kidney disease progresses.
Practical relevance It is important to recognize the presence of anemia of renal disease so that adequate treatment may be instituted to improve quality of life and metabolic function. Erythrocyte-stimulating agents (ESAs), such as epoetin alfa, epoetin beta and darbepoetin alfa, have been developed to counteract the effects of decreased erythropoietin production by the kidneys. These treatments, which are the focus of this review, have 83% similarity in amino acid sequence to the feline hormone. On average, the target packed cell volume (>25%) is reached within 3–4 weeks of ESA therapy.
Clinical challenges The use of ESAs has been associated with a number of complications, such as iron deficiency, hypertension, arthralgia, fever, seizures, polycythemia and pure red cell aplasia (PRCA). Darbepoetin has a prolonged half-life compared with epoetin and thus can be given only once a week, instead of three times a week. The incidence of PRCA appears to be decreased with darbepoetin use when compared with epoetin use in cats.
Evidence base There is limited published evidence to date to underpin the use of ESAs in cats. This review draws on the relevant publications that currently exist, and the authors’ personal experience of using these therapies for over 5 years.
Anemia — a quality of life issue
Chronic kidney disease (CKD) is a common disorder of domestic cats. Previous studies have indicated that 15–30% of geriatric cats may develop some degree of renal insufficiency or overt azotemia. 1,2 The kidneys perform multiple important metabolic and endocrine functions in the body, including contributing to erythropoiesis. It is estimated that 30–65% of cats with CKD develop anemia as their renal disease worsens (Fig 1). 1,3–5 In addition to lack of appropriate erythropoiesis, uremia from progressing renal disease can decrease red blood cell (RBC) survival.
FIG 1.
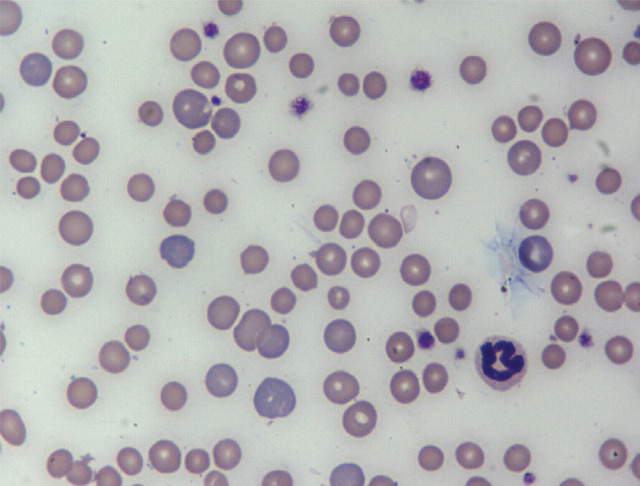
The anemia of CKD is typically normocytic, normochromic and non-regenerative. In this blood smear from a cat with longstanding anemia of CKD, acute gastrointestinal hemorrhage and iron deficiency, the RBCs demonstrate anisocytosis (variable sizes), polychromasia (younger RBCs have more grayish-blue color) and central pallor. Hypochromia is not commonly seen in cats compared with dogs with iron deficiency anemia. Wright-Giemsa, ×100. Courtesy of Andrea Siegel, ALX Laboratory
There is contradictory evidence as to whether anemia represents a predictor of survival in cats with CKD, but certainly chronic anemia affects the body negatively in a number of ways, leading to an overall decrease in quality of life. 2,6,7 It is, therefore, important to recognize and diagnose anemia of renal disease and to eliminate other causes of anemia so that appropriate therapy may be implemented.
An estimated 30–65% of cats with CKD develop anemia as their renal disease worsens.
Erythropoietin's role in erythropoiesis
Erythropoiesis is controlled by the production of the hematopoietic growth factor erythropoietin in response to anemia. 8,9 Erythropoietin is mainly produced in the peritubular interstitial cells of the inner renal cortex and outer medulla in the kidney. The liver, brain, uterus, peripheral endothelial cells, muscles and insulin-producing cells also produce erythropoietin, but to a much lesser degree. 8–11 As kidney disease progresses, there are fewer erythropoietin-producing cells within the kidneys. 1,9,11
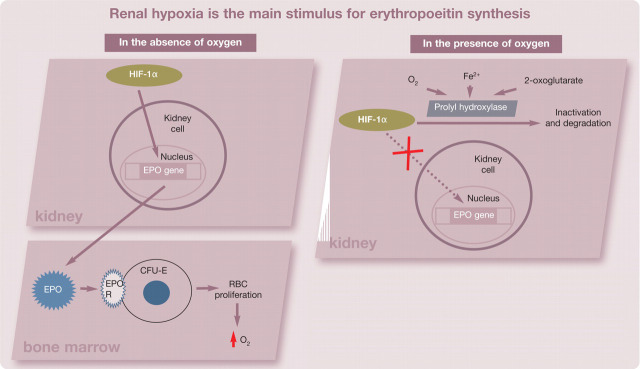
The main stimulus for erythropoietin synthesis is renal hypoxia. When hypoxia is present, degradation of hypoxia-inducible factor 1 (HIF-1α) is inhibited, and HIF-1α is free to bind to hypoxia-response elements of oxygen-regulated genes (Fig 1). These response elements control the erythropoietin gene within the kidneys, and binding of HIF-1α stimulates an increase in production of erythropoietin. 12–14 The rate of production of erythropoietin is inversely proportional to the oxygen-carrying capacity of blood. 9 The main site of erythropoietin action is the bone marrow, where it binds to its receptor expressed on the surface of erythroid progenitor cells and leads to increased erythropoiesis (Fig 2). 11 Erythropoietin has the greatest influence on colony-forming unit-erythron (CFU-E) cells; these cells contain the largest number of erythropoietin receptors. 15
FIG 1.
In the absence of oxygen, HIF-1α increases transcription of the erythropoeitin (EPO) gene, creating more EPO. EPO binds to its receptor on colony-forming unit-erythron (CFU-E) cells in the bone marrow and prevents apoptosis, enhancing proliferation and maturation to RBCs, which increase oxygen-carrying capacity. When oxygen is present, prolyl hydroxylase is stabilized and increases degradation of HIF-1α, preventing EPO production. HIF-1 = hypoxia-inducible factor 1, EPO R = erythropoeitin receptor
FIG 2.
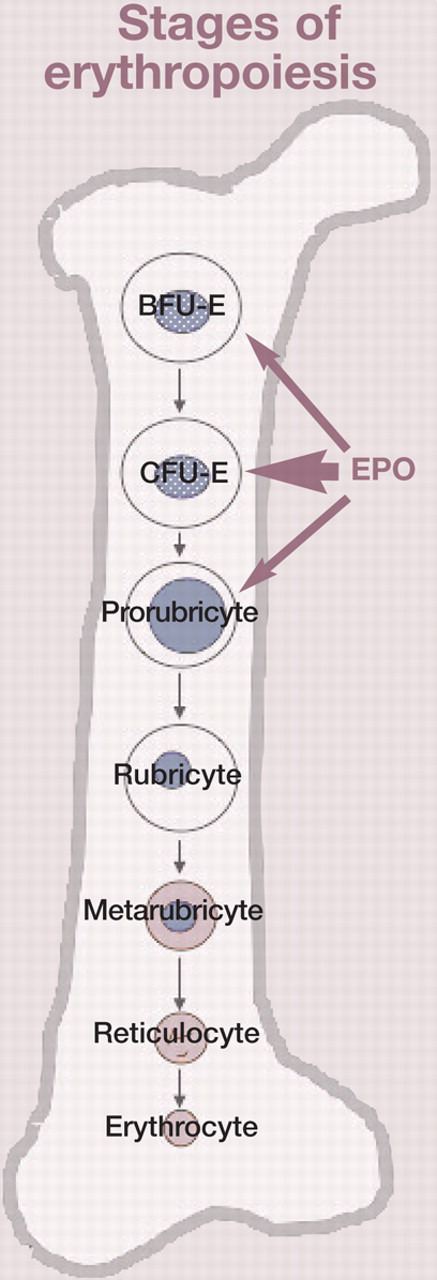
Colony-forming unit-erythron (CFU-E) cells have the highest number of erythropoietin (EPO) receptors. Blast-forming unit-erythron (BFU-E) cells and prorubricytes are also capable of responding to EPO
Anemia and its consequences
Anemia is defined as a state of deficient mass of circulating RBCs and hemoglobin, which results in reduced oxygen delivery to all organs and a subsequent decline in cell metabolism. 16,17 Anemia triggers numerous adaptive response mechanisms due to tissue hypoxia, some of which may be detrimental in the long term. Adaptive mechanisms include increased release of plasma norepinephrine, renin, angiotensin II and aldosterone, all of which can lead to increased sympathetic nervous system response, increased heart contractility and rate, and increased blood pressure. 18
As anemia becomes more severe, cats may also develop left heart enlargement secondarily to hemodynamic compensation, and become more prone to congestive heart failure, especially if receiving intravenous fluid therapy. 19
Pathogenesis of anemia of renal disease
Anemia of renal disease is multifactorial in its pathogenesis (see top box, page 631). The lack of erythropoeitin is the main cause of anemia in CKD patients. 20 Additional factors contributing to anemia of renal disease include anemia of inflammation, other causes of suppressed erythropoiesis, the influence of uremic toxins on RBC survival, and gastrointestinal hemorrhage or other sources of blood loss.
Causes of anemia in CKD.
Decreased erythropoiesis
Lack of erythropoietin
Inflammatory cytokines
Absolute iron deficiency
Functional iron deficiency
Uremic toxins
ACE inhibitors, angiotensin receptor antagonists
Hyperparathyroidism
Marrow fibrosis/infiltration
Shortened RBC survival
Uremic toxins
Hemolysis
Premature removal by reticuloendothelial cells
Increased RBC loss
Thrombocytopathy
Gastrointestinal ulcers
Blood sampling
Decreased erythropoiesis
Anemia of inflammation (both acute and chronic) contributes to anemia of renal disease, and CKD itself is known to be associated with a chronic pro-inflammatory state. 21 Cytokines such as interferon-α, -β and -γ, as well as tumor necrosis factor-α, interleukin-1 and interleukin-6, are produced in inflammatory states and create a relative iron deficiency by induction of hepcidin, an acute phase protein. 21,22
Control of hepcidin production.
Production is stimulated by:
Iron presence
Inflammation
Chronic kidney disease
Production is suppressed by:
Iron deficiency
Erythropoeitic activity
Hepcidin is considered the central regulator of systemic iron homeostasis.
Hepcidin is produced by the liver in response to inflammation and iron loading, and is suppressed by normal erythropoietic activity and iron deficiency. Hepcidin is considered to be the central regulator of systemic iron homeostasis and the primary inflammatory mediator causing its increased production is interleukin-6. 22,23
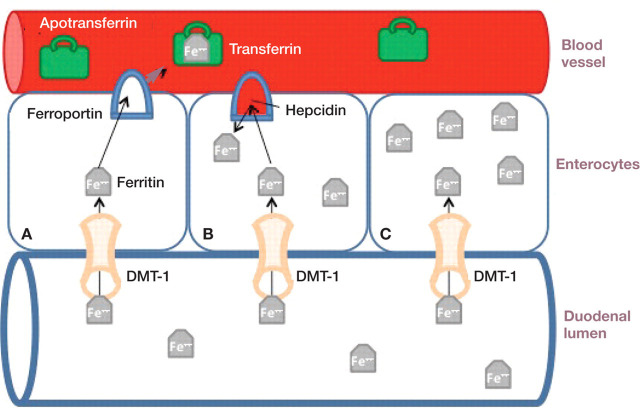
Hepcidin's biological actions are mediated by its binding to and internalization of ferroportin, which is the principal cellular efflux channel for iron. Orally ingested iron is absorbed into the duodenal enterocytes via the apical protein DMT-1, and enters the circulation via ferroportin on the basolateral side (Fig 3). Hepcidin traps iron in the enterocytes by enhancing degradation of ferroportin. Hepcidin prevents release of iron stored in macrophages and hepatocytes as well. 23,24 The decrease in enteral absorption and the sequestration of iron in macrophages eventually leads to anemia by decreasing the availability of iron for hemoglobin production. Hepcidin production is suppressed in the face of active erythropoiesis, which enhances availability of iron for hemoglobin production, but the mechanism by which this happens is poorly understood. 24 Hepcidin concentrations are increased in human CKD patients, even in those without significant inflammation, in part due to decreased renal clearance. 22,24
FIG 3.
Ingested iron is absorbed from the duodenal lumen into the enterocyte through the divalent metal transporter (DMT-1) on the luminal side. In the absence of hepcidin, iron leaves the enterocyte on the basolateral side via ferroportin (cell A). Apotransferrin bound to iron is called transferrin, and is the main method of transporting iron in the circulation. Hepcidin binds to ferroportin, causing rapid internalization and degradation of ferroportin (cell B). Without ferroportin, iron efflux from the cell is prevented (cell C)
In addition to induction of hepcidin production, the aforementioned inflammatory cytokines can negatively affect RBC survival and directly decrease responsiveness to erythropoietin, all contributing to worsening anemia. 21,25
Several uremic inhibitors of erythropoiesis have also been identified. However, their relative contribution to anemia is controversial. It is thought that each of these substances affects erythropoiesis at various stages within the bone marrow. 26,27 Secondary hyperparathyroidism as a consequence of CKD can cause erythropoietin hyporesponsiveness. Parathyroid hormone in excess may have a direct toxic effect on erythroid progenitor cells, and also an indirect effect by inducing bone marrow fibrosis. 28 Deficiency of B vitamins may also impair erythropoiesis.
Common therapies used for CKD may impair RBC production. Angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers are suspected to decrease angiotensin II-induced release of erythropoietin, as well as prevent pluripotent hemopoietic stem cell recruitment. 28,29 Aluminum toxicity, from aluminum containing phosphate binders, can interfere with iron metabolism, leading to a microcytic anemia that is not responsive to iron administration. 30–33
Lack of erythropoeitin is the main cause of anemia in CKD patients.
Shortened RBC survival
Uremia has been associated with decreased RBC survival, but the pathophysiology is unclear and most likely multifactorial. 34 There is suspicion of a uremic toxin circulating and affecting RBC lifespan. In part, the decreased survival is due to increased low-grade hemolysis of RBCs as uremia progresses. 35,36 In addition, there is evidence that lipid peroxidation of red cell membranes may occur as uremia progresses, resulting in premature clearance by the reticuloendothelial system, which is activated in the inflammatory state. 37
Increased RBC loss
Blood loss and blood sampling contribute to the anemia of CKD. Uremia affects platelets and can lead to uremic thrombocytopathy. This may be due to retention of small substances normally eliminated by functioning kidneys that affect platelet function, vascular damage caused by uremic toxins, changes in platelet responsiveness and function, changes in platelet-endothelial interactions, increased levels of nitric oxide (a potent platelet antagonist) in uremic states, and dysregulation of coagulation factors responsible for normal platelet function. 38–40 Manifestations of platelet dysfunction include gastrointestinal bleeding, bleeding from surgical sites and mucocutaneous bleeding. 41
Significant blood loss can occur through the gastrointestinal tract in people with CKD in the absence of identifiable discrete bleeding ulcers. 42,43 Studies of the prevalence of upper gastrointestinal ulceration in humans with CKD yield conflicting results, although recent evidence suggests that CKD patients do not have a higher prevalence of ulceration compared with patients with normal kidney function. 43–46 Although gastrin, a hormone responsible for stimulating hydrochloric acid secretion in the stomach, is cleared by the kidneys, and gastrin concentration in the blood is elevated in people and cats with CKD, hyperacidity and gastric ulceration are variably present. 47–49 Gastric pathology is common in dogs with kidney disease, but ulceration is uncommon; 50 similar studies in cats are lacking. Acute upper gastrointestinal bleeding may manifest as an acute onset of anemia, hypoproteinemia, hypovolemia or hypotension.
Therapy with erythrocyte-stimulating agents (ESAs)
Human erythropoietin is a 30,400 Dalton glycosylated protein that contains a 165 amino acid residue backbone and has a half-life of approximately 6–10 h. 51 The gene sequence for erythropoietin was discovered in 1983, which allowed for the production of recombinant erythropoietin in 1985. 52 Recombinant human erythropoietin (rHuEPO) products have an identical amino acid sequence to the natural hormone. 15,53–55 Multiple recombinant erythropoietin products are available, including epoetin alfa (Epogen, Amgen; Procrit, Centocor Ortho Biotech Products; Eprex, Janssen), epoetin beta (Neo-Recormon, Roche), darbepoetin alfa (Aranesp, Amgen) and continuous erythropoietin receptor activators (Mircera, Roche). These vary in their degree of glycosylation, but there is no difference in their clinical efficacy. 56 The amount of glycosylation affects renal clearance, thus influencing the frequency of administration. 57
Canine erythropoietin shares an 81.3% homology with the amino acid sequence of human erythropoietin, while homology in cats is 83.3%. 58,59 The relative conservation of amino acid sequence allows for rHuEPO products to bind to and interact with erythropoietin receptors of dogs and cats.
Epoetin
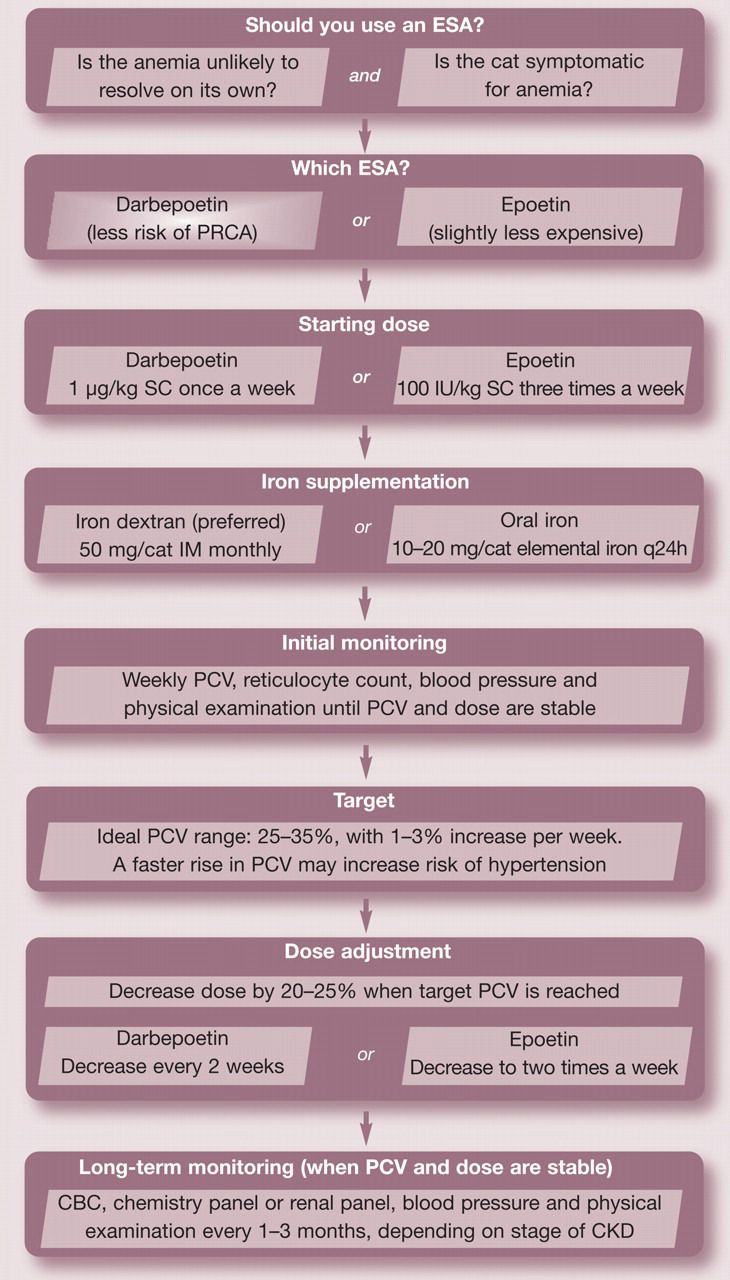
Epoetin was the first ESA used in client-owned pets with naturally occurring CKD. The usual starting dose of 100 IU/kg administered subcutaneously three times a week is recommended until the packed cell volume (PCV) reaches the low end of the target range (see guidelines, page 633). 60 The authors recommend a target PCV of ≥25% in cats. A response is usually seen within 3–4 weeks. Once the target range is attained, maintenance doses of 50–100 IU/kg twice weekly are usually sufficient. Supplementation of iron at the initiation of and during epoetin therapy is also recommended to ensure an adequate response to the ESA.
Darbepoetin
Darbepoetin, a hyperglycosylated recombinant human erythropoietin analogue, was developed in the early 1990s, and differs from epoetin in that it contains five N-linked carbohydrate chains. 61,62 The development of the molecule was based on the hypothesis that adding an N-linked carbohydrate chain — thus increasing the amount of sialic acid residues — would result in a molecule with a longer circulating half-life. 60,63 By increasing the half-life, darbepoetin could be administered less often. Although data is not available in cats, pre-clinical studies of darbepoetin in dogs demonstrated a threefold increase in half-life when compared with epoetin (25.0 vs 7.2 h), and a correspondingly reduced mean clearance rate (2.4 ml/kg/h vs 8.4 ml/kg/h). 62 Extensive human studies evaluating the use of darbepoetin to correct anemia of renal disease in CKD patients have demonstrated clinical efficacy. The proportion of patients achieving a satisfactory hemoglobin concentration has been similar to those patients receiving epoetin. 62,64–67
The authors have been using darbepoetin alfa as the sole ESA therapy for anemia of renal disease for many years with good success.
A darbepoetin dose of 0.45 μg/kg once weekly administered subcutaneously has been demonstrated to provide optimal responses in 60–70% of human patients over a 4-week period; 62,68 an optimal response in people is defined as a hemoglobin increase of 1–3 g/dl over the first 4 weeks. There are no published effective dosages for companion animals. Based on the authors’ database, 12% of cats responded adequately to a starting dose of 0.45 μg/kg once weekly, whereas 62% responded at a dose of 1.0 μg/kg once weekly. Thus, we recommend a starting dose of 1.0 μg/kg once weekly until the target PCV is attained, thereafter decreasing the frequency of administration to every 2–3 weeks. On average, a response is expected within 2–3 weeks. The dose is then adjusted to maintain the PCV within the target range (25–35%).
The authors have been using darbepoetin as the sole ESA therapy for anemia of renal disease for many years with a good success rate. Feline recombinant erythropoietin has also been developed, but studies have demonstrated that the incidence of pure red cell aplasia (PRCA), a complication associated with the production of neutralizing antibodies, was not significantly reduced when compared with the incidence in cats administered epoetin. 69
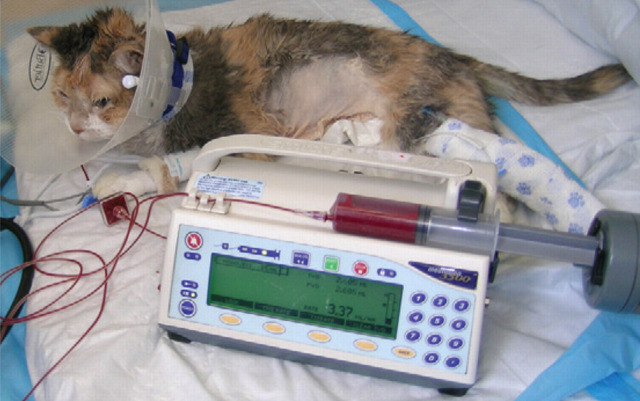
We recommend monitoring the PCV weekly (Fig 4) until both the PCV and dosing regimen are stable. In addition, a reticulocyte count should be evaluated weekly to ensure adequate bone marrow stimulation until the maintenance phase of therapy is reached, and thereafter monthly. However, it has been our experience that reticulocyte counts of cats receiving darbepoetin often do not reflect the effectiveness of therapy (ie, the PCV reaches target, but the reticulocyte count may not increase). We speculate that as darbepoetin is administered once weekly, the rise in reticulocyte count occurring in the few days after darbepoetin administration may be missed when evaluated 7 days after administration. Blood pressure should also be assessed at each examination (Fig 5) as hypertension is one of the most common side effects of ESA therapy.
FIG 4.

The PCV should be monitored weekly at the start of ESA therapy. Frequency of monitoring can be decreased once the PCV is stable and ESA dose adjustments are no longer needed
FIG 5.
Because hypertension is a reported side effect of ESA therapy, blood pressure should be monitored at each visit, especially during the initial phases of treatment when the PCV is rising
Guidelines for using ESAs.
Iron supplementation
Lack of available iron for erythropoiesis is recognized as the leading cause of treatment failure in humans on ESA therapy. 70–72 Iron is necessary for hemoglobin formation and RBC function, and should be administered to all cats receiving ESAs. Cats with CKD that responded to treatment with feline erythropoietin had a decrease in mean cell volume serum iron concentration and percentage transferrin saturation despite receiving oral iron supplementation. 69 The decrease in circulating iron parameters during ESA therapy may represent utilization of iron for RBC production in excess of intake or in excess of available iron stores, inadequate intestinal iron absorption despite oral iron supplementation, or functional iron deficiency due to sequestration of iron in storage sites such as the bone marrow with an inability to utilize that iron due to alterations in hepcidin metabolism. 20,69
There is a state of relative iron deficiency in many cats with CKD during ESA therapy. Evaluation of serum iron parameters is recommended at the start of ESA therapy and monthly thereafter. Cats with true iron deficiency would be expected to have low serum iron, ferritin and transferrin saturation. In cases of chronic inflammation (including CKD patients), ferritin and total iron binding capacity may be normal or above the reference interval, whereas serum iron and transferrin saturation should be below the reference interval (see below).
True iron deficiency or chronic inflammation?
Iron deficiency
Low serum iron
Low ferritin
Low total iron binding capacity
Low % saturation
Chronic inflammation
Low serum iron
High ferritin
Normal-high total iron binding capacity
Low % saturation
Iron should be administered to all cats receiving ESA therapy.
Oral iron supplements, such as ferrous sulfate compounds, tend to be poorly absorbed in the gastrointestinal system, and the bitter taste may cause some cats to reject them. The recommended dosage is 50–100 mg/cat per day administered orally (10–20 mg of elemental iron). A popular oral liquid multivitamin (Pet-Tinic; Pfizer) contains 12.5 mg of elemental iron per 5 ml. Tablet formulations of iron typically contain anywhere from 35–100 mg of elemental iron. Iron dextran given by intramuscular injection every 3–4 weeks along with an ESA may be a better alternative to oral iron supplementation. The dose of iron dextran is typically 50 mg/cat. Intramuscular injection can be painful, but iron dextran should not be administered intravenously as there is a risk of anaphylaxis.
Iron therapy in patients with chronic inflammation is considered controversial. Iron is an essential nutrient for microorganisms and its sequestration may be a defense mechanism against infection, and iron promotes formation of toxic hydroxyl radicals. 25,73
Other treatments
Anabolic steroids
The use of nandrolone and stanzolol has been reported in the human literature for erythrocyte stimulation, but their efficacy is doubtful and hepatic side effects are common. 74–78 These products are not recommended for treating anemia of CKD in cats.
Transfusion therapy
Transfusion of whole blood or packed RBC preparations is indicated when there is acute blood loss or when a patient has severe clinical signs of anemia (Fig 6). Disadvantages of blood transfusions include the possibility of immune reactions and donor—recipient incompatibility, the limited availability of blood products, the reduced lifespan of infused blood products in a uremic patient, the associated costs of blood product transfusion, and the lack of long-term effectiveness of these products. 5
FIG 6.
Transfusion of whole blood or packed RBCs is the most rapid way to correct anemia of CKD and may be indicated if clinical signs of anemia need urgent treatment or if aggressive intravenous fluid therapy is anticipated and congestive heart failure is a concern
Oxyglobin
Oxyglobin is a hemoglobin-based oxygen carrier that is a sterile solution of purified, polymerized bovine hemoglobin and a modified lactated Ringer's buffer. It has a lower viscosity than blood and has enhanced oxygen offloading characteristics. 79 Its main indications are for temporary oxygen-carrying capacity in anemia caused by hemorrhage or inadequate erythropoiesis. Effects are short-lived, cost is a limiting factor, and circulatory overload is a concern in cats with concurrent cardiac disease. 80
Complications of ESA therapy.
The use of ESAs can create a number of complications, such as iron deficiency, hypertension, arthralgia, fever, seizures, polycythemia and PRCA. In humans, it is known that darbepoetin has a similar adverse event profile to that of epoetin. 62 Hypertension is reported in 23% of those administered darbepoetin, with seizures in 2%, and cerebrovascular disorders and PRCA in less than 1% each. 85
Hypertension
Hypertension is a side effect of epoetin administration in 40–50% of dogs and cats. 57,86 Based on the authors’ experience, similar percentages are seen with darbepoetin therapy. The pathophysiology of hypertension with ESA therapy includes an increase in blood viscosity, improved cardiac output, reduced vasodilation as compensation for anemia, and imbalance in vasoactive hormones with increased vascular responsiveness. 73 Uncontrolled hypertension may contribute to the development of seizures in cats treated with darbepoetin.
Pure red cell aplasia
PRCA is caused by the production of neutralizing anti-erythropoietin antibodies that cross-react with all ESAs, as well as with endogenous erythropoietin. 87–89 It is characterized by a severe, non-regenerative anemia with an almost complete lack of bone marrow RBC precursors. The antibodies can persist for over 8 months, and patients with PRCA become transfusion dependent. 5 The cytological definition of PRCA varies significantly in the veterinary literature.
The use of ESAs can create a number of complications, such as iron deficiency, hypertension, arthralgia, fever, seizures, polycythemia and PRCA.
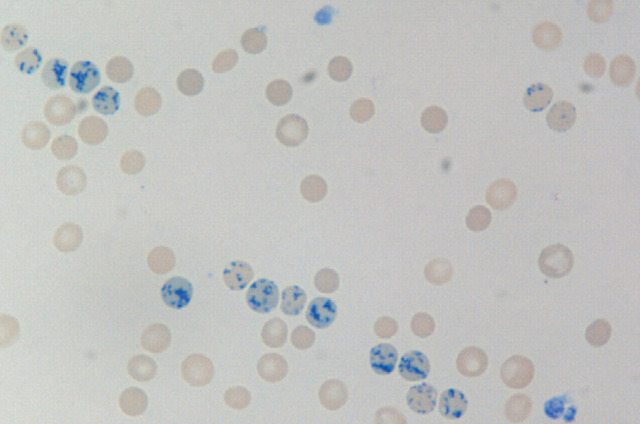
New methylene blue stained blood smear from a cat receiving darbepoetin showing multiple reticulocytes (RBCs with speckled blue stain uptake). Detection of reticulocytosis may be affected by sample timing. A decrease in reticulocyte count in conjunction with a decrease in PCV may portend development of PRCA. ×100. Courtesy of Andrea Siegel, ALX Laboratory
Myeloid to erythroid (M:E) ratios of 4:1 to 299:1 have been reported in dogs and cats with PRCA. 20,57,86 Antibody-mediated PRCA is an extremely rare occurrence in humans, with fewer than 300 cases reported worldwide, the majority of which have been associated with use of epoetin. 89 In contrast, PRCA is an important concern in companion animals using human-based ESAs. As mentioned, the amino acid sequence of canine and feline erythropoietin shares approximately 83% homology with rHuEPO. Anti-erythropoietin antibodies are directed against the protein backbone, and this lack of complete homology is thought to be the cause of the increased rate of PRCA in animals compared with humans. 90
PRCA seems to occur at an incidence of 25–30% in dogs and cats receiving epoetin. 86,91 In the experience of the authors, the occurrence of PRCA seems to be much lower with darbepoetin compared with epoetin, with a frequency of less than 10%.
PRCA is difficult to treat and requires immediate discontinuation of ESA administration, as well as possible institution of immunosuppressive therapy. There is no established best immunosuppressive protocol in humans or animals. 92,93 With repeated transfusions, identifying cross-match compatible blood becomes more difficult and, even with transfusion of cross-matched blood, the rate of RBC destruction seems to accelerate, and many cats need weekly transfusions. Because of quality of life concerns, as well as financial implications, the development of PRCA leads many owners to consider euthanasia.
This product is no longer available in the United States.
Vitamins and L-carnitine
Commercially prepared kidney diets contain adequate vitamin supplementation, making vitamin deficiency unlikely in a patient consuming an amount adequate to maintain body weight. However, partial anorexia is common in cats with CKD. Several B vitamins are necessary for erythropoiesis, including cobalamin (B12), folic acid (B9), niacin (B3) and pyridoxine (B6). Deficiencies of these vitamins may exacerbate anemia and increase erythropoietin resistance. Although there is a paucity of data on whether CKD patients commonly develop deficiencies of these vitamins, supplementation of these water soluble vitamins is unlikely to cause harm, but they are not effective as sole therapies for anemia. 81,82
L-carnitine is believed to increase CFU-E cells (see Fig 2) and stimulate erythropoiesis. Although L-carnitine deficiency is common in people on long-term hemodialysis, and there is a modest benefit of L-carnitine supplementation in these patients if they are erythropoietin-resistant, there is little evidence that pre-dialysis patients are L-carnitine deficient. 83,84 There exists no data describing L-carnitine status in cats with CKD.
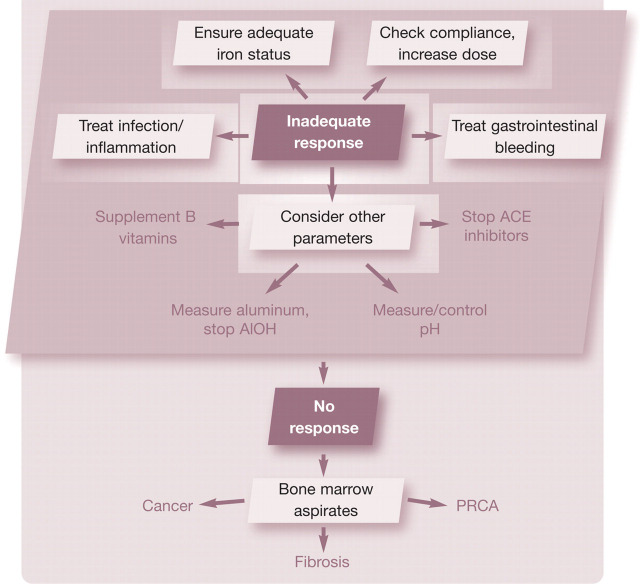
Treatment failure with ESAs
Failure to reach the target PCV during ESA therapy can be due to a number of factors (Table 1). Over 90% of people receiving ESA therapy respond adequately. 62 However, based on the authors’ experience of treating over 70 cats with epoetin or darbepoetin, only about 60–65% had an adequate response. In humans, a decreased response to ESA therapy is defined as a significant decrease in hemoglobin level at a constant ESA dose, a significant increase in ESA dose to preserve a certain hemoglobin level, or a failure to raise the hemoglobin level to greater than 11 g/dl (110 g/l) despite an ESA dose equivalent to greater than 500 IU/kg a week of epoetin or 1.5 μg/kg a week of darbepoetin. 29
The approach to a cat with an inadequate response to ESA therapy involves evaluation of multiple parameters (see box on the right). The order of evaluation may vary based on the clinical picture, and several parameters may be evaluated simultaneously. Iron deficiency is present in 25–38% of anemic people with CKD and is the leading cause of failure to achieve target hematocrit in people on ESA therapy 28,29 Changes in the complete blood count (low mean cell volume, low mean cell hemoglobin content) are not sensitive indicators of iron deficiency. 94 Serum iron parameters (total iron, total iron binding capacity, ferritin) can be difficult to interpret in the face of concurrent infection, inflammation or functional iron deficiency.
Infection and inflammation are common disorders in people with CKD and can have a dramatic impact on erythropoiesis and erythropoietin resistance. As discussed earlier, inflammatory cytokines directly suppress erythropoiesis and decrease iron availability by upregulation of hepcidin production. A thorough search for infectious complications (ie, urinary tract infection, dental infection, feeding tube exit site infection) and aggressive treatment may restore responsiveness to ESA.
TABLE 1.
Causes of inadequate ESA response described in people
| Iron deficiency (absolute or functional) |
| Infection/inflammation |
| Chronic blood loss |
| Osteitis fibrosis |
| Aluminum toxicity |
| Hemoglobinopathy (sickle cell anemia, β-thalassemia) |
| Folate or vitamin B12 deficiency |
| Multiple myeloma |
| Malnutrition |
| Hemolysis |
| Dialysis-related carnitine deficiency |
| PRCA |
| ACE inhibitors/angiotensin receptor antagonists |
| Inadequate dialysis dose/frequency |
Inadequat eresponse to ESA therapy?
Based on the authors’ experience of treating over 70 cats with epoetin or darbepoetin, only about 60–65% of cats had an adequate response.
In some cases, the ESA dose may be too low for the patient or be incorrectly administered. A higher ESA dose may partially overcome ESA resistance, although doses higher than 500 IU/kg a week for epoetin or 1.5–2.0 μg/kg a week of darbepoetin are rarely used by the authors.
If the PCV does not improve after addressing these issues, other reasons for hypo-responsiveness (deficiency of B vitamins, hyperparathyroidism, aluminum toxicity, inhibition of erythropoiesis by ACE inhibitors) should be investigated and addressed. If the PCV still remains low, a bone marrow aspirate or core biopsy should be considered to rule out occult neoplasia, bone marrow fibrosis or PRCA.
Future directions
Darbepoetin has become the mainstay for treatment of anemia of renal disease in cats. However, use of this drug does not come without risks and complications. Newer drugs in development may carry less risk and be more effective. An oral prolyl hydroxylase inhibitor is under development for humans and companion animals. This drug prevents degradation of HIF-1α (see Fig 1), thus enhancing native erythropoietin production. 95,96 There is also a need for diagnostic assays that allow for better characterization of anemia of renal disease, so that treatment may be tailored to the individual needs of each patient. The development of a hepcidin assay that is consistent and reliable may prove useful in determining iron status and aid in iron management. 97
Case notes.
Bobby is a 12-year-old castrated male domestic shorthair cat who was diagnosed with International Renal Interest Society stage III CKD when he was presented for polyuria and polydipsia. He had a blood urea nitrogen (BUN) of 71 mg/dl (25.3 mmol/l), creatinine of 4.5 mg/dl (398 μmol/l), hematocrit of 29.4% and a systolic blood pressure of 220 mmHg. Treatments started at that time included switching to a renal diet, every other day subcutaneous fluid therapy and oral amlodipine. His blood pressure normalized at 140 mmHg. On a routine recheck 8 months after his initial presentation, the owners were happy with his clinical status at home. However, he had lost 0.5 kg, his BUN was 60 mg/dl (21.4 mmol/l), his creatinine was 3.3 mg/dl (292 μmol/l), his hematocrit was 21% and his reticulocyte count was 18,200/μl.
Ongoing symptomatic treatment Given the lack of clinical signs of anemia, Bobby's treatments were not changed at that time, but the owners were instructed to return him for a recheck examination in a month. At the time of this recheck, he had lost an additional 0.2 kg, had a BUN of 68 mg/dl (24 mmol/l), a creatinine of 3.5 mg/dl (309 μmol/l), a hematocrit of 17.9% and a reticulocyte count of 4180/μl. The following week, his BUN was 95 mg/dl (34 mmol/l), his creatinine was 3.7 mg/dl (327 μmol/l), his hematocrit was 15.1% and his reticulocyte count was 4660/μl.
As the progressive increase in the BUN to creatinine ratio may suggest dehydration or gastrointestinal bleeding, treatment with gastroprotectants (ie, sucralfate and famotidine) was initiated. Although treatment to stop blood loss from an ulcer may allow the anemia to correct itself, the rate of improvement can be very slow in patients with CKD. The following day, Bobby was presented for weakness, with a hematocrit of 13.6%, a reticulocyte count of 3200/μl and a systolic blood pressure of 105 mmHg.
Given the rapid decrease in PCV and the appearance of clinical signs associated with anemia, a transfusion of one unit of packed RBCs was administered, increasing the PCV to 19%.
ESA therapy Treatment with darbepoetin was initiated at a dose of 1 μg/kg once a week subcutaneously. The PCV the following week was 24%, the reticulocyte count was 36,790/μl and all other clinical parameters remained stable. Two weeks after starting darbepoetin, the PCV decreased to 21%. On week 3 of therapy, the PCV was 18% and his reticulocyte count was 4250/μl.
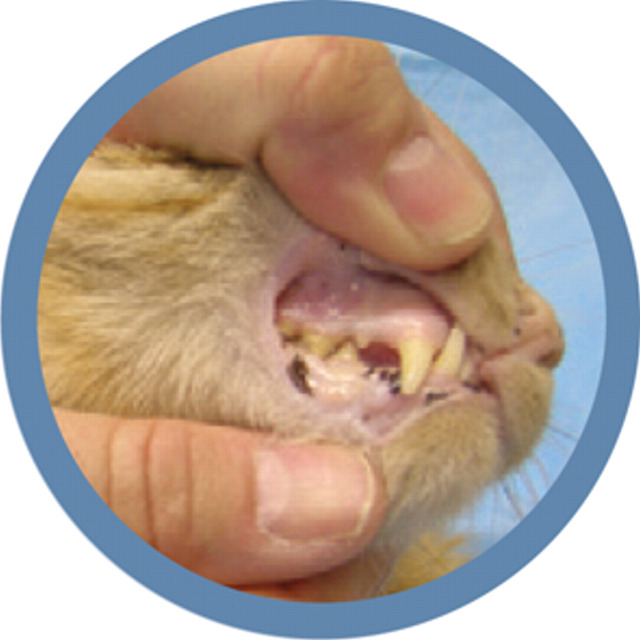
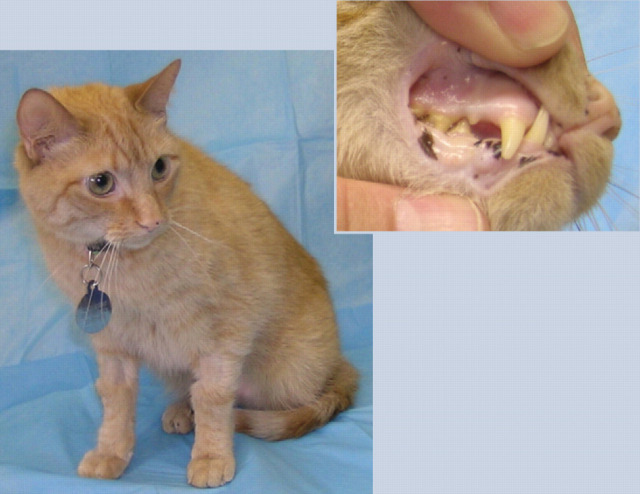
Bobby's pale mucous membranes are a classic sign of anemia
Chronic gastrointestinal blood loss can cause iron deficiency. A serum iron panel was submitted and 50 mg of iron dextran was administered intramuscularly. The dose of darbepoetin was also increased to 2 μg/kg once weekly. The dose increase was made to overcome erythropoietin resistance, despite concerns that the decrease in PCV may have been due to PRCA. The following week, the PCV had increased to 22%, and the reticulocyte count increased to 16,560/μl; at this time another 2 μg/kg dose of darbepoetin was administered. The results of the serum iron panel showed hypoferremia (18 μg/dl, reference interval [RI] 33–134 μg/dl; 3.2 μmol/l, RI 6–24 μmol/l), low % saturation (6%, RI 20–33%), and a high ferritin (399 ng/ml, RI 31–144 ng/ml; 896.6 pmol/l, RI 70–324 pmol/l), suggesting functional iron deficiency associated with inflammation.
Bobby's PCV the following week was 29%, and his darbepoetin dose was decreased to 1 μg/kg once weekly. A further week on, the PCV was 33%, so the darbepoetin dose was decreased again, to 1 μg/kg every other week. The PCV stabilized between 27 and 32% on a dose of 1 μg/kg administered every other week.
WHAT THIS CASE DEMONSTRATES
Bobby's case provides a typical example of renal anemia developing in a cat with CKD, and illustrates the active follow-up necessary to reach and maintain target PCV.
KEY POINTS.
Anemia related to kidney disease is multifactorial in its pathogenesis; however, the main cause is decreased erythropoietin production by the failing kidneys.
A large percentage of cats with kidney disease will develop anemia.
Acute and chronic inflammation contributes to anemia of renal disease by the production of inflammatory cytokines and substances such as hepcidin that will decrease erythropoietin function, red cell survival and available iron.
Multiple therapies for treating anemia of renal disease exist. However, the use of erythrocyte-stimulating agents (ESAs), such as darbepoetin or epoetin, and elimination of other potential causes of anemia, seem to be most efficient in the long term.
While both darbepoetin and epoetin can be effective in reversing anemia of renal disease, darboetin has a longer half-life and is less likely to cause pure red cell aplasia when compared with epoetin in cats.
Iron is essential for hemoglobin formation and red cell function, and should be administered to all cats receiving ESA therapy.
References
- 1. Lulich JP, Osborne CA, O'Brien TD, Polzin DJ. Feline renal failure: questions, answers, questions. Compend Contin Educ Pract Vet 1992; 14: 127–53. [Google Scholar]
- 2. Jepson RE, Brodbelt D, Vallance C, Syme HM, Elliott J. Evaluation of predictors of the development of azotemia in cats. J Vet Intern Med 2009; 23: 806–13. [DOI] [PubMed] [Google Scholar]
- 3. Elliott J, Barber PJ. Feline chronic renal failure: clinical findings in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract 1998; 39: 78–85. [DOI] [PubMed] [Google Scholar]
- 4. DiBartola SP, Rutgers HC, Zack PM, Tarr MJ. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973–1984). J Am Vet Med Assoc 1987; 190: 1196–202. [PubMed] [Google Scholar]
- 5. Cowgill LD. Pathophysiology and management of anemia in chronic progressive renal failure. Semin Vet Med Surg (Small Anim) 1992; 7: 175–82 [PubMed] [Google Scholar]
- 6. King J, Tasker S, Gunn-Moore D, Strehlau G, Group BS. Prognostic factors in cats with chronic kidney disease. J Vet Intern Med 2007; 21: 906–16. [PubMed] [Google Scholar]
- 7. Boyd LM, Langston CE, Thompson K, Zivin K, Imanishi M. Survival in cats with naturally occurring chronic kidney disease (2000–2002). J Vet Intern Med 2008; 22: 1111–17 [DOI] [PubMed] [Google Scholar]
- 8. Maiese K, Chong ZZ, Shang YC. Raves and risks of erythropoietin. Cytokine Growth Factor Rev 2008; 19: 145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Erslev AJ, Besarab A. Erythropoietin in the pathogenesis and treatment of the anemia of chronic renal failure. Kidney Int 1997; 51: 622–30. [DOI] [PubMed] [Google Scholar]
- 10. Eschbach JW, Adamson JW. Anemia of end-stage renal disease (ESRD). Kidney Int 1985; 28: 1–5. [DOI] [PubMed] [Google Scholar]
- 11. Eschbach JW. The anemia of chronic renal failure: pathophysiology and the effects of recombinant erythropoietin. Kidney Int 1989; 35: 134–48. [DOI] [PubMed] [Google Scholar]
- 12. Semenza GL, Agani F, Booth G, et al. Structural and functional analysis of hypoxia-inducible factor 1. Kidney Int 1997; 51: 553–55. [DOI] [PubMed] [Google Scholar]
- 13. Semenza G. Regulation of erythropoietin production: new insights into molecular mechanism of oxygen homeostasis. Hematol Oncol Clin North Am 1994; 8: 863–64. [PubMed] [Google Scholar]
- 14. Zhu H, Bunn HF. Oxygen sensing and signaling: impact on the regulation of physiologically important genes. Respir Physiol 1999; 115: 239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fisher JW. Erythropoietin: physiology and pharmacology update. Exp Biol Med 2003; 228: 1–14. [DOI] [PubMed] [Google Scholar]
- 16. Brazier J, Cooper N, Maloney JV, Jr, Buckberg G. The adequacy of myocardial oxygen delivery in acute normovolemic anemia. Surgery 1974; 75: 508–16. [PubMed] [Google Scholar]
- 17. Cain SM. Oxygen delivery and uptake in dogs during anemic and hypoxic hypoxia. J Appl Physiol 1977; 42: 228–34. [DOI] [PubMed] [Google Scholar]
- 18. Fishbane SB, Barry M. Hematologic aspects of kidney disease. In: Brenner BM, ed. Brenner & Rector's the kidney. 8th edn. Philadelphia: Saunders Elsevier, 2008: 1733–44. [Google Scholar]
- 19. Wilson HE, Jasani S, Wagner TB, et al. Signs of left heart volume overload in severely anaemic cats. J Feline Med Surg 2010; 12: 904–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Randolph JF, Scarlett JM, Stokol T, MacLeod JN. Clinical efficacy and safety of recombinant canine erythropoietin in dogs with anemia of chronic renal failure and dogs with recombinant human erythropoietin-induced red cell aplasia. J Vet Intern Med 2004; 18: 81–91. [DOI] [PubMed] [Google Scholar]
- 21. Stenvinkel P. The role of inflammation in the anaemia of endstage renal disease. Nephrol Dial Transplant 2001; 16 (suppl 7): 36–40. [DOI] [PubMed] [Google Scholar]
- 22. Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol 2009; 122: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 2004; 113: 1271–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Young B, Zaritsky J. Hepcidin for clinicians. Clin J Am Soc Nephrol 2009; 4: 1384–87. [DOI] [PubMed] [Google Scholar]
- 25. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005; 352: 1011–23. [DOI] [PubMed] [Google Scholar]
- 26. Brunati C, Cappellini MD, De Feo T, et al. Uremic inhibitors of erythropoiesis: a study during treatment with recombinant human erythropoietin. Am J Nephrol 1992; 12: 9–13. [DOI] [PubMed] [Google Scholar]
- 27. Radtke HW, Rege AB, LaMarche MB, et al. Identification of spermine as an inhibitor of erythropoiesis in patients with chronic renal failure. J Clin Invest 1981; 67: 1623–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kwack C, Balakrishnan VS. Managing erythropoietin hypo-responsiveness. Semin Dial 2006; 19: 146–51. [DOI] [PubMed] [Google Scholar]
- 29. Kanbay M, Perazella MA, Kasapoglu B, Koroglu M, Covic A. Erythropoiesis stimulatory agent-resistant anemia in dialysis patients: review of causes and management. Blood Purif 2010; 29: 1–12. [DOI] [PubMed] [Google Scholar]
- 30. Segev G, Bandt C, Francey T, Cowgill LD. Aluminum toxicity following administration of aluminum-based phosphate binders in two dogs with renal failure. J Vet Intern Med 2008; 22: 1432–35. [DOI] [PubMed] [Google Scholar]
- 31. Priyadarshi A, Shapiro JI. Erythropoietin resistance in the treatment of the anemia of chronic renal failure. Semin Dial 2006; 19: 273–78. [DOI] [PubMed] [Google Scholar]
- 32. Jeffery EH, Abreo K, Burgess E, Cannata J, Greger JL. Systemic aluminum toxicity: effects on bone, hematopoietic tissue, and kidney. J Toxicol Environ Health 1996; 48: 649–65. [DOI] [PubMed] [Google Scholar]
- 33. Cannata-Andia JB, Fernandez-Martin JL. The clinical impact of aluminium overload in renal failure. Nephrol Dial Transplant 2002; 17 (suppl 2): 9–12. [DOI] [PubMed] [Google Scholar]
- 34. Erslev AJ, Besarab A. The rate and control of baseline red cell production in hematologically stable patients with uremia. J Lab Clin Med 1995; 126: 283–86. [PubMed] [Google Scholar]
- 35. Naets JP. Hematologic disorders in renal failure. Nephron 1975; 14: 181–94. [DOI] [PubMed] [Google Scholar]
- 36. Bonomini M, Sirolli V. Uremic toxicity and anemia. J Nephrol 2003; 16: 21–28. [PubMed] [Google Scholar]
- 37. Miguel A, Miguel A, Linares M, et al. Evidence of an increased susceptibility to lipid peroxidation in red blood cells of chronic renal failure patients. Nephron 1988; 50: 64–65. [DOI] [PubMed] [Google Scholar]
- 38. Linthorst GE, Avis HJ, Levi M. Uremic thrombocytopathy is not about urea. J Am Soc Nephrol 2010; 21: 753–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boccardo P, Remuzzi G, Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost 2004; 30: 579–89. [DOI] [PubMed] [Google Scholar]
- 40. Boyle JM, Johnston B. Acute upper gastrointestinal hemorrhage in patients with chronic renal disease. Am J Med 1983; 75: 409–12. [DOI] [PubMed] [Google Scholar]
- 41. Himmelfarb J. Hematologic manifestations of renal failure. In: Greenberg A, ed. Primer on kidney diseases. 4th edn. Philadelphia: Elsevier Saunders, 2005: 519–26. [Google Scholar]
- 42. Rosenblatt SG, Drake S, Fadem S, Welch R, Lifschitz MD. Gastrointestinal blood loss in patients with chronic renal failure. Am J Kidney Dis 1982; 1: 232–36. [DOI] [PubMed] [Google Scholar]
- 43. Karagiannis S, Goulas S, Kosmadakis G, et al. Wireless capsule endoscopy in the investigation of patients with chronic renal failure and obscure gastrointestinal bleeding (preliminary data). World J Gastroenterol 2006; 12: 5182–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stolic RV, Jovanovic AN, Zivic ZP, et al. Influence of the level of renal insufficiency on endoscopic changes in the upper gastrointestinal tract. Am J Med Sci 2008; 336: 39–43. [DOI] [PubMed] [Google Scholar]
- 45. Abu Farsakh NA, Roweily E, Rababaa M, Butchoun R. Brief report: evaluation of the upper gastrointestinal tract in uraemic patients undergoing haemodialysis. Nephrol Dial Transplant 1996; 11: 847–50. [DOI] [PubMed] [Google Scholar]
- 46. Shirazian S, Radhakrishnan J. Gastrointestinal disorders and renal failure: exploring the connection. Nat Rev Nephrol 2010; 6: 480–92. [DOI] [PubMed] [Google Scholar]
- 47. Goldstein RE, Marks SL, Kass PH, Cowgill LD. Gastrin concentrations in plasma of cats with chronic renal failure. J Am Vet Med Assoc 1998; 213: 826–28. [PubMed] [Google Scholar]
- 48. Ciccotosto GD, Dawborn JK, Hardy KJ, Shulkes A. Gastrin processing and secretion in patients with end-stage renal failure. J Clin Endocrinol Metab 1996; 81: 3231–38. [DOI] [PubMed] [Google Scholar]
- 49. Kes P. Serum gastrin concentration in chronic renal failure. Acta Med Croatica 1992; 46: 47–58. [PubMed] [Google Scholar]
- 50. Peters RM, Goldstein RE, Erb HN, Njaa BL. Histopathologic features of canine uremic gastropathy: a retrospective study. J Vet Intern Med 2005; 19: 315–20. [DOI] [PubMed] [Google Scholar]
- 51. Car B. Erythropoiesis and erythrokinetics. In: Feldman BF, Zinkl JG, Jain NC, eds. Schalm's veterinary hematology. 5th edn. Philadelphia: Lippincott, Williams & Wilkins, 2000: 105–9. [Google Scholar]
- 52. Bunn H. Erythropoietin: current status. Yale J Biol Med 1990; 63: 381–86. [PMC free article] [PubMed] [Google Scholar]
- 53. Jelkman W. Molecular biology of erythropoietin. Intern Med 2004; 43: 649–59. [DOI] [PubMed] [Google Scholar]
- 54. Porter D, Goldberg M. Physiology of erythropoietin production. Semin Hematol 1994; 31: 112–21. [PubMed] [Google Scholar]
- 55. Egrie JC, Strickland TW, Lane J, et al. Characterization and biological effects of recombinant human erythropoietin. Immunobiology 1986; 172: 213–24. [DOI] [PubMed] [Google Scholar]
- 56. Storring PL, Tiplady RJ, Gaines Das RE, et al. Epoetin alfa and beta differ in their erythropoietin isoform compositions and biological properties. Br J Haematol 1998; 100: 79–89. [DOI] [PubMed] [Google Scholar]
- 57. Langston CE, Reine NJ, Kittrell D. The use of erythropoietin. Vet Clin North Am Small Animal Pract 2003; 33: 1245–60. [DOI] [PubMed] [Google Scholar]
- 58. MacLeod JN. Species-specific recombinant erythropoietin preparations for companion animals. In: Proceedings of 2001 ACVIM Veterinary Medical Forum; 2001; Denver, CO: 578–79.
- 59. MacLeod JN, Tetreault JW, Lorschy KAS, Gu DN. Expression and bioactivity of recombinant canine erythropoietin. Am J Vet Res 1998; 59: 1144–48. [PubMed] [Google Scholar]
- 60. Egrie JC, Dwyer E, Browne JK, Hitz A, Lykos MA. Darbepoetin alfa has a longer circulation half-life and greater in vivo potency than recombinant human erythropoietin. Exp Hematol 2003; 31: 290–99. [DOI] [PubMed] [Google Scholar]
- 61. Macdougall IC, Matcham J, Gray S. Correction of anaemia with darbepoetin alfa in patients with chronic kidney disease receiving dialysis. Nephrol Dial Transplant 2003; 18: 576–81. [DOI] [PubMed] [Google Scholar]
- 62. Macdougall IC. Darbepoetin alfa: a new therapeutic agent for renal anemia. Kidney Int 2002; 61 (suppl 80): S55–S61. [DOI] [PubMed] [Google Scholar]
- 63. Harris D, Collins J, Disney A, et al. Darbepoetin alfa: a new erythropoietic drug for the treatment of renal anaemia. Nephrol 2002; 7: S173–S80. [Google Scholar]
- 64. Brunkhorst R, Bommer J, Braun J, et al. Darbepoetin alfa effectively maintains haemoglobin concentrations at extended dose intervals relative to intravenous or subcutaneous recombinant human erythropoietin in dialysis patients. Nephrol Dial Transplant 2004; 19: 1224–30. [DOI] [PubMed] [Google Scholar]
- 65. Locatelli F, Canaud B, Giacardy F, Martin-Malo A, Baker N, Wilson J. Treatment of anaemia in dialysis patients with unit dosing of darbepoetin alfa at a reduced dose frequency relative to recombinant human erythropoietin (rHuEpo). Nephrol Dial Transplant 2003; 18: 362–69. [DOI] [PubMed] [Google Scholar]
- 66. Toto RD, Pichette V, Navarro J, et al. Darbepoetin alfa effectively treats anemia in patients with chronic kidney disease with de novo every-other-week administration. Am J Nephrol 2004; 24: 453–60. [DOI] [PubMed] [Google Scholar]
- 67. Carrera F, Oliveira L, Maia P, Mendes T, Ferreira C. The efficacy of intravenous darbepoetin alfa adminstered once every 2 weeks in chronic kidney disease patients on haemodialysis. Nephrol Dial Transplant 2006; 21: 2846–50. [DOI] [PubMed] [Google Scholar]
- 68. Macdougall IC. Novel erythropoiesis-stimulating agents: a new era in anemia management. Clin J Am Soc Nephrol 2008; 3: 200–7. [DOI] [PubMed] [Google Scholar]
- 69. Randolph JF, Scarlett JM, Stokol T, Saunders KM, MacLeod JN. Expression, bioactivity, and clinical assessment of recombinant feline erythropoietin. Am J Vet Res 2004; 65: 1355–66. [DOI] [PubMed] [Google Scholar]
- 70. Macdougall IC. Strategies for iron supplementation: oral versus intravenous. Kidney Int 1999; 55 (suppl 69): S61–S6. [DOI] [PubMed] [Google Scholar]
- 71. Schiesser D, Binet I, Tsinalis D, et al. Weekly low-dose treatment with intravenous iron sucrose maintains iron status and decreases epoetin requriement in iron-replete haemodialysis patients. Nephrol Dial Transplant 2006; 21: 2841–45. [DOI] [PubMed] [Google Scholar]
- 72. Sjaastad OV, Framstad T, Blom AK. Effect of iron on erythropoietin production in anaemic piglets. Acta Vet Scand 1996; 37: 133–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fishbane S, Masani N. Anemia in chronic kidney disease. In: Pereira BJG, Sayegh MH, Blake P, eds. Chronic kidney disease, dialysis, and transplantation. 2nd edn. Philadelphia: Elsevier Saunders, 2005: 122–35. [Google Scholar]
- 74. Shahani S, Braga-Basaria M, Maggio M, Basaria S. Androgens and erythropoiesis: past and present. J Endocrinol Invest 2009; 32: 704–16. [DOI] [PubMed] [Google Scholar]
- 75. Johansen KL. The role of nandrolone decanoate in patients with end stage renal disease in the erythropoietin era. Int J Artif Organs 2001; 24: 183–85. [PubMed] [Google Scholar]
- 76. Dainiak N. The role of androgens in the treatment of anemia of chronic renal failure. Semin Nephrol 1985; 5: 147–54. [PubMed] [Google Scholar]
- 77. Basaria S, Wahlstrom JT, Dobs AS. Clinical review 138: anabolic-androgenic steroid therapy in the treatment of chronic diseases. J Clin Endocrinol Metab 2001; 86: 5108–17. [DOI] [PubMed] [Google Scholar]
- 78. Harkin KR, Cowan LA, Andrews GA, et al. Hepatotoxicity of stanozolol in cats. J Am Vet Med Assoc 2000; 217: 681–84. [DOI] [PubMed] [Google Scholar]
- 79. Callan MB, Rentko VT. Clinical application of a hemoglobin-based oxygen-carrying solution. Vet Clin North Am Small Anim Pract 2003; 33: 1277–93, vi. [DOI] [PubMed] [Google Scholar]
- 80. Weingart C, Kohn B. Clinical use of a haemoglobin-based oxygen carrying solution (Oxyglobin) in 48 cats (2002–2006). J Feline Med Surg 2008; 10: 431–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Don T, Friedlander S, Wong W. Dietary intakes and biochemical status of B vitamins in a group of children receiving dialysis. J Ren Nutr 2010; 20: 23–28. [DOI] [PubMed] [Google Scholar]
- 82. Druml W. Nutritional support in patients with acute renal failure. In: Molitoris BA, Finn WF, eds. Acute renal failure: a companion to Brenner and Rector's the kidney. Philadelphia: WB Saunders; 2001: 465–89. [Google Scholar]
- 83. Fouque D, Holt S, Guebre-Egziabher F, et al. Relationship between serum carnitine, acylcarnitines, and renal function in patients with chronic renal disease. J Ren Nutr 2006; 16: 125–31. [DOI] [PubMed] [Google Scholar]
- 84. Stivelman JC, Fishbane S, Nissenson AR. Erythropoietin therapy in renal disease and renal failure. In: Brenner BM, ed. Brenner & Rector's the kidney. 8th edn. Philadelphia: Saunders Elsevier; 2008: 1884–1903. [Google Scholar]
- 85. Weiss G. Pathogenesis and treatment of anaemia of chronic disease. Blood Rev 2002; 16: 87–96. [DOI] [PubMed] [Google Scholar]
- 86. Cowgill LD, James KM, Levy JK, et al. Use of recombinant human erythropoietin for management of anemia in dogs and cats with renal failure. J Am Vet Med Assoc 1998; 212: 521–28. [PubMed] [Google Scholar]
- 87. Pollock C, Johnson DW, Horl WH, et al. Pure red cell aplasia induced by erythropoiesis-stimulating agents. Clin J Am Soc Nephrol 2008; 3: 193–99. [DOI] [PubMed] [Google Scholar]
- 88. Macdougall IC. An overview of the efficacy and safety of novel erythropoiesis stimulating protien (NESP). Nephrol Dial Transplant 2001; 16 (suppl 3): 14–21. [DOI] [PubMed] [Google Scholar]
- 89. Macdougall IC. Antibody-mediated pure red cell aplasia (PCRA): epidemiology, immunogenicity and risks. Nephrol Dial Transplant 2005; 20 (suppl 4): iv9–iv15. [DOI] [PubMed] [Google Scholar]
- 90. Wen D, Boissel JP, Tracy TE, et al. Erythropoietin structure—function relationships: high degree of sequence homology among mammals. Blood 1993; 82: 1507–16. [PubMed] [Google Scholar]
- 91. Cowgill LD. Application of recombinant human erythropoietin in dogs and cats. In: Kirk RW, Bonagura JD, eds. Current veterinary therapy XI. Philadelphia: WB Saunders, 1992: 484–87. [Google Scholar]
- 92. Bennett CL, Cournoyer D, Carson KR, et al. Long-term outcome of individuals with pure red cell aplasia and antierythropoietin antibodies in patients treated with recombinant epoetin: a follow-up report from the Research on Adverse Drug Events and Reports (RADAR) Project. Blood 2005; 106: 3343–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rossert J, Macdougall IC, Casadevall N. Antibody-mediated pure red cell aplasia (PRCA) treatment and re-treatment: multiple options. Nephrol Dial Transplant 2005; 20 (suppl 4): iv23–iv6. [DOI] [PubMed] [Google Scholar]
- 94. Steinberg JD, Olver CS. Hematologic and biochemical abnormalities indicating iron deficiency are associated with decreased reticulocyte hemoglobin content (CHr) and reticulocyte volume (rMCV) in dogs. Vet Clin Pathol 2005; 34: 23–27. [DOI] [PubMed] [Google Scholar]
- 95. Bernhardt WM, Wiesener MS, Scigalla P, et al. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J Am Soc Nephrol 2010; 21: 2151–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Owen WE, Roberts WL. Performance characteristics of a new Immulite 2000 system erythropoietin assay. Clin Chim Acta 2011; 412: 480–82. [DOI] [PubMed] [Google Scholar]
- 97. Schwarz P, Strnad P, von Figura G, et al. A novel monoclonal antibody immunoassay for the detection of human serum hepcidin. J Gastroenterol 2011; 46: 648–56. [DOI] [PubMed] [Google Scholar]