Abstract
Thirteen cases of feline primary hyperaldosteronism were diagnosed based on clinical signs, serum biochemistry, plasma aldosterone concentration, adrenal imaging and histopathology of adrenal tissue. Two cases presented with blindness caused by systemic hypertension, whilst the remaining 11 cases showed weakness resulting from hypokalaemic polymyopathy. Elevated concentrations of plasma aldosterone and adrenocortical neoplasia were documented in all cases. Seven cases had adrenal adenomas (unilateral in five and bilateral in two) and six had unilateral adrenal carcinomas. Three cases underwent medical treatment only with amlodipine, spironolactone and potassium gluconate; two cases survived for 304 and 984 days until they were euthanased because of chronic renal failure, whilst the third case was euthanased at 50 days following failure of the owner to medicate the cat. Ten cases underwent surgical adrenalectomy following a successful stabilisation period on medical management. Five cases remain alive at the time of writing with follow-up periods of between 240 and 1803 days. Three cases were euthanased during or immediately following surgery because of surgical-induced haemorrhage. One cat was euthanased 14 days after surgery because of generalised sepsis, whilst the remaining cat was euthanased 1045 days after surgery because of anorexia and the development of a cranial abdominal mass. It is recommended that primary hyperaldosteronism should be considered as a differential diagnosis in middle-aged and older cats with hypokalaemic polymyopathy and/or systemic hypertension and should no longer be considered a rare condition.
Aldosterone is the major mineralocorticoid secreted by the zona glomerulosa of the adrenal gland in response to stimulation of the renin–angiotensin–aldosterone system (RAAS) and has primary effects on mineralocorticoid receptors in the distal renal tubules, colon and salivary glands to stimulate sodium reabsorption, and potassium and hydrogen excretion (Stewart 1999). Sodium retention results in an expansion of the extracellular fluid volume. The clinical signs of primary hyperaldosteronism typically result from systemic hypertension caused by blood volume expansion and/or polymyopathy due to hypokalaemia. Primary hyperaldosteronism (PHA) was first reported in humans in 1955 (Conn 1955) and is important in man as a potentially curable form of hypertension. The term secondary hyperaldosteronism (SHA) was coined to describe other conditions, such as congestive cardiac failure and hepatic cirrhosis, in which aldosterone production is a normal adrenal response to the activation of the RAAS (Conn et al 1964).
Primary hyperaldosteronism is currently regarded as a rare condition (Ahn 1994, Feldman and Nelson 2004) with only two canine (Breitschwerdt et al 1985, Rijnberk et al 2001a) and six feline cases reported in the literature. Eger et al (1983) reported a 17-year-old domestic shorthair (DSH) cat with generalised weakness caused by profound hypokalaemia. Hypertension was not documented in this case but the pupils were dilated and unresponsive to light. MacKay et al (1999) reported a 5-year-old normotensive domestic longhair cat with episodic weakness associated with hypokalaemia. Flood et al (1999) reported two DSH cases, aged 20 and 10 years, which were both hypertensive and hypokalaemic, although muscular weakness was not a feature. Moore et al (2000) diagnosed PHA in a 13-year-old DSH cat with hypokalaemia-induced muscle weakness, ataxia and dysphagia, whilst Rijnberk and others (2001b) reported a 12-year-old DSH presenting with muscular weakness.
This paper describes 13 previously unreported cases of feline PHA, including two cases of bilateral adrenal adenomas previously not reported in cats. The large number of cases reported, together with long follow-up times, allows a detailed profile of the condition to be described in a case series for the first time.
Materials and methods
Nine cases (cases 1–8 and 12) were presented to a first opinion small animal practice, Grove Lodge Veterinary Hospital, between 1997 and 2004 and four cases (cases 9–11 and 13) were referred to the University of Bristol Veterinary School between 1999 and 2003. All cases underwent full clinical examination, routine haematology and an extensive biochemistry profile. Creatine kinase (CK) was measured in 11 cases. Thyroxine concentration was measured in nine cases. Plasma aldosterone concentration (PAC) was measured in all cases. Systolic arterial blood pressure (BP) was measured in 12 cases using Doppler-shift sphygmomanometry (Parkes model 811-BTS, Parks Medical, Perimed) with a 9.5 MHz probe using a previously described method (Sparkes et al 1999). Repeated readings≥170 mmHg were considered to indicate hypertension. Abdominal ultrasonography to visualise the adrenal glands was performed in 11 cases either conscious or under sedation with intramuscular ketamine (Ketaset injection; Fort Dodge Animal Health) and diazepam (Valium; Roche). Abdominal magnetic resonance imaging (MRI), using 3 mm slices, was performed in two cases under general anaesthesia using intravenous propofol (Rapinovet; Schering-Plough Animal Health).
Surgical adrenalectomy was performed or attempted in 10 cases. An intravenous infusion (0.08–0.25 mmol/kg/h) of potassium chloride (Potassium Forte; Martindale Pharmaceuticals) was administered before anaesthesia for adrenalectomy to maintain serum potassium concentrations between 3.5 and 4.5 mmol/l. Anaesthesia was induced with intravenous propofol following premedication with intramuscular acepromazine (C-Vet Veterinary Products) and buprenorphine (Vetergesic; Reckitt and Coleman) in nine cases, and acepromazine and morphine (Martindale Pharmaceuticals) in three cases. Anaesthesia was maintained with inhalational isoflurane USP (Iso-flo: Schering-Plough Animal Health). Laparotomies were performed by a ventral midline approach to allow exposure of both adrenal glands and other organs (Birchard 1993). Care was taken to maintain the adrenal capsule intact where possible during dissection for adenalectomy, to avoid fragmentation of the tumour and possible seeding of neoplastic cells into the peritoneal cavity, and adrenalectomy was performed as described previously (Birchard 1993). Following adrenalectomy samples of excised tissue were submitted for histopathology.
Results
Signalment and clinical presentation
Ten cats were DSH, with one Siamese, one Burmese and one Burmilla. All where neutered; eight males and five females. The age of the cats ranged from 6 to 13 years with a mean age of the 10 years. Two of the 13 cases in this series presented with sudden onset blindness caused by intraocular haemorrhage (IOH) and retinal detachment, and 11 cases presented with polymyopathy. The two cases presenting with IOH showed no evidence of clinical myopathy, whilst three of the polymyopathy cases showed evidence of hypertensive retinopathy on fundic examination but had no vision abnormalities. Six of the 11 cases with polymyopathy presented with acute onset of severe weakness. Case 4 had presented with episodic forelimb stiffness for a couple of months before acute collapse occurred, and case 8 had some dysphagia 2 weeks before acute onset of cervical ventroflexion and paresis. Cases 9, 11 and 13 had a history of polyuria and polydipsia before muscle weakness was observed. Additionally cases 11 and 13 showed polyphagia. Polymyopathy signs comprised cervical ventroflexion in seven cases, hindlimb weakness and pain in three cases, and paresis in three cases (Table 1).
Table 1.
Clinical and laboratory features of 13 cases of feline hyperaldosteronism*
| Case no. | Breed | Age (yrs) | Sex | Clinical presentation | K | Na | CK | Urea | Creat | TT4 | U SG | BP (mmHg) | PAC (pmol/l) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DSH | 13 | FN | Blindness. Bilateral retinal detachment. No evidence of polymyopathy. | ↓ | N | N | ↑ | N | N | 1.034 | 200 | 877 |
| 2 | DSH | 13 | FN | Blindness. Bilateral retinal detachment. No evidence of polymyopathy. | ↓ | N | nd | N | N | N | nd | 200 | 1551 |
| 3 | DSH | 8 | MN | Polymyopathy. No ocular signs. | ↓↓ | N | ↑↑ | ↑ | N | nd | 1.037 | 180 | 3158 |
| 4 | Siamese | 8 | MN | Forelimb stiffness then generalised polymyopathy. No ocular signs. | ↓↓ | N | ↑↑ | N | N | nd | 1.018 | 180 | 3240 |
| 5 | DSH | 8 | MN | Polymyopathy. No ocular signs. | ↓↓ | N | ↑ | N | N | nd | nd | nd | 1634 |
| 6 | DSH | 10 | MN | Polymyopathy. Increased retinal vessel tortuosity on fundic examination. | ↓ | N | ↑↑ | N | ↑ | N | nd | 250 | 3712 |
| 7 | DSH | 12 | FN | Polymyopathy. No ocular signs. | ↓↓ | N | ↑↑ | ↑↑ | ↑ | ↓ | 1.038 | 190 | 8005 |
| 8 | Burmese | 13 | FN | Mild dysphagia. Polymyopathy. No ocular signs. | ↓↓ | N | ↑ | N | N | nd | nd | 170 | 13 850 |
| 9 | DSH | 9 | MN | PU/PD. Polymyopathy. No ocular signs. | ↓ | N | ↑↑ | N | N | N | 1.010 | 160 | 4464 |
| 10 | DSH | 6 | MN | Polymyopathy. No ocular signs. | ↓↓ | N | ↑ | ↑ | ↑ | N | 1.040 | 170 | 6230 |
| 11 | Burmilla | 10 | FN | PU/PD/PP. Polymyopathy. No ocular signs. | ↓ | N | ↑ | ↑ | N | N | 1.039 | 170 | 6480 |
| 12 | DSH | 12 | MN | Polymyopathy. Bullous retinopathy on fundic examination. | ↓↓ | N | nd | ↑ | N | N | 1.022 | 195 | 14 653 |
| 13 | DSH | 12 | MN | PU/PD/PP. Polymyopathy. Bullous retinopathy on fundic examination. | ↓↓ | ↑ | ↑ | ↑↑ | N | ↑ | 1.022 | 250 | 7800 |
DSH=domestic shorthair; FN=female neutered; MN=male neutered; PU=polyuria; PD=polydipsia; PP=polyphagia; K=potassium concentration; Na=sodium concentration; CK=creatine kinase concentration; Urea=urea concentration; Creat=creatinine concentration; TT4 = total thyroxine concentration; USG=urine specific gravity; BP=systolic blood pressure; PACUSG=plasma aldosterone concentration;
↓ below reference range; ↓↓ far below reference range (≤2.5 mmol/l for K); ↑ above reference range; ↑↑ far above reference range (≥1000 iu/l for CK, ≥20 mmol/l for urea); N=normal; nd=not done.
Haematology and biochemistry
No significant abnormalities were found on haematological profiles at initial presentation. Hypokalaemia was present in all cases at initial presentation with a mean value of 2.5 mmol/l (ranging from 1.9 to 3.2; reference range 4–5.5). In all but one case, sodium concentration was within the reference range (mean 155 mmol/l, ranging from 148 to 168; reference range 140–160). CK was elevated in all of the 10 polymyopathy cases in which it was measured (mean 6837 IU/l, ranging from 209 to 56 240; reference range<120) and was normal (80) in the IOH case in which it was measured. Urea concentration was elevated in seven cases and ranged from 4.9 to 25.3 mmol/l with a mean value of 11.2 (reference range 6.5–10). Creatinine concentration was elevated in three cases and ranged from 86 to 278 μmol/l with a mean value of 121 (reference range<175). Thyroxine concentration ranged from 13 to 92 nmol/l with a mean value of 33 (reference range 19–65), being normal in seven cases, reduced in one case and elevated in one case. Urine specific gravity was measured at presentation in nine cases and ranged from 1.010 to 1.040 with a mean value of 1.029. Eleven cases were hypertensive and one was normotensive. Elevated PAC was measured in all cases ranging from 877 to 14 653 pmol/l with a mean value of 5820 (reference range 150–430) (Table 1).
Imaging
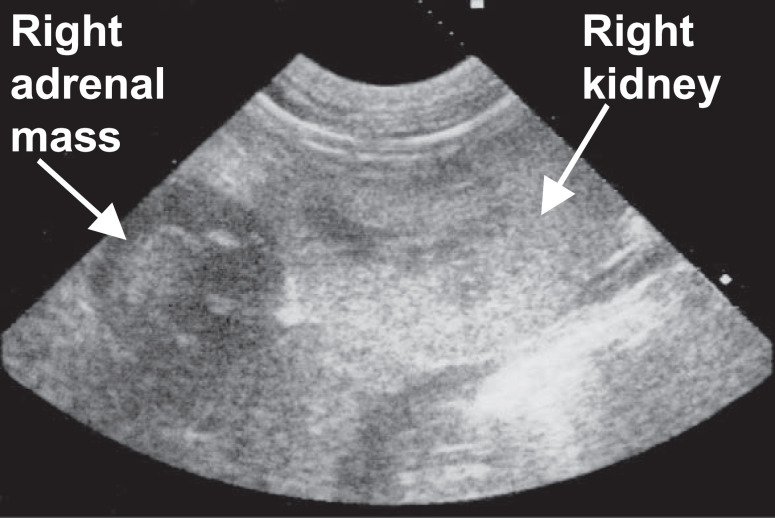
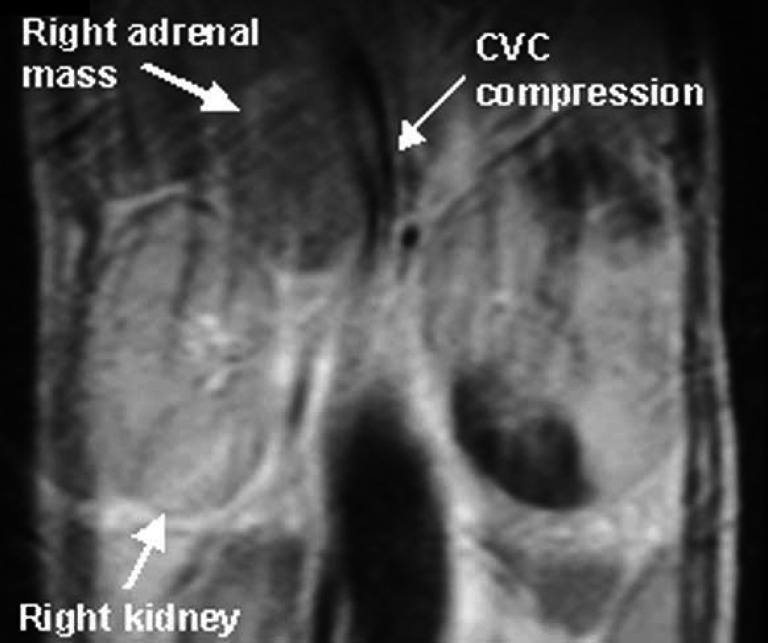
Adrenal ultrasonography revealed unilateral adrenal enlargement with evidence of an adrenal mass in all 11 cases in which it was performed (Fig 1). The contralateral adrenal was either not identified or described as being normal. One case (case 9) found to have unilateral adrenal enlargement on ultrasonography was subsequently found to have bilateral adrenal adenomas on post mortem examination 13 days later. The other cat (case 7) found to have bilateral adrenal adenomas on post mortem examination did not have ultrasonography performed. Measurement of the adrenal glands was performed in only six cases and unilateral enlargement ranged from 1 to 3.5 cm by 1 to 2.5 cm in size. In cases 9 and 10 a close association between the adrenal mass and caudal vena cava (CVC) was identified by ultrasonography. In cases 5 and 10 MRI was performed in an attempt to establish the extent of the adrenal mass before undertaking surgery. Both cases showed compression of the CVC by an adrenal mass but no obvious extension of the mass into the lumen of the CVC (Fig 2) (Table 2).
Fig 1.
Abdominal ultrasound sonogram in case 5 showing a hypoechoic right adrenal mass cranial to the right kidney. Histopathology showed this adrenal mass to be an adrenocortical carcinoma.
Fig 2.
Close up of a sagittal section of the MRI scan of case 5 at the level of adrenal mass showing compression of the caudal vena cava (CVC).
Table 2.
Ultrasonographical, treatment and histopathological features of 13 cases of feline hyperaldosteronism*
| Case no. | Adrenal ultrasonography | AC pathology | Surgery† | Outcome | Survival† |
| 1 | R 3×1 cm hypoechoic; L not seen | R C | N | Good response to K, spiron and amlo but no return of vision. Euthanasia due to severe chronic renal failure. | Euthanasia d304 |
| 2 | nd | L A | N | K, spiron and amlo but BP remained high with no return of vision. Euthanasia due to severe chronic renal failure. | Euthanasia d984 |
| 3 | R enlarged and hypoechoic, not measured; L not seen | R A | Y on d70 | Good response to K, spiron and amlo before surgery. Healthy at time of writing. | Alive at d1803 |
| 4 | R 2.1×1.7 cm hypoechoic; L not seen | R A | Y on d40 | Good response to K, spiron and amlo before surgery. Healthy at time of writing. | Alive at d1278 |
| 5 | R enlarged and hypoechoic, not measured; L not seen | R C | Y on d20 | Good response to K and spiron before surgery. Euthanasia due to anorexia and cranial abdominal mass but PME not performed. | Euthanasia d1065 |
| 6 | R adrenal not seen; L enlarged but not measured | L A | Y on d75 | Good response to K, spiron and amlo before surgery. On treatment for chronic renal failure. | Alive at d500 |
| 7 | nd | Bilat A | N | Good initial response to K, spiron and amlo but lack of owner compliance for treatment after 7 days. Euthanasia due to severe weakness. | Euthanasia d50 |
| 8 | R 2×2 cm hypoechoic; L N | R C | Y on d14 | Good response to K and spiron before surgery. Healthy at time of writing | Alive at d431 |
| 9 | R 3.5×2.5 cm adhered to liver and CVC; L N | Bilat A | Y on d13 | Good response to K and spiron before surgery. Euthanasia due to severe H+ at surgery. | Euthanasia d13 |
| 10 | R enlarged and compressing dorsal suface CVC, not measured; L N | R A | Y on d149 | Good response to K and spiron before surgery. Euthanasia due to severe H+ immediately post surgery. | Euthanasia d149 |
| 11 | R enlarged but not measured; LN | R C | Y on d183 | Good response to K and spiron before surgery. Euthanasia due to severe H+ immediately post surgery. | Euthanasia d183 |
| 12 | R adrenal N; L 2×2 cm hypoechoic | L C | Y on d30 | Good response to K, spiron and amlo before surgery. Healthy at time of writing | Alive at d240 |
| 13 | R not identified; L 1×1 cm hypoechoic | L C | Y on d30 | Good response to K, spiron, amlo and carbimazole before surgery, although BP remained high. Did well after surgery but generalised sepsis developed 14 days after surgery leading to euthanasia. | Euthanasia d44 |
L=left; R=right; cm=centimeters; CVC=caudal vena cava; AC=adrenocortical; C=carcinoma; A=adenoma; Bilat=bilateral; N=no; Y=yes; d=days; K=potassium supplementation; spiron=spironolactone; amlo=amlodipine; H+=haemorrhage; nd=not done.
Value given refers to number of days after initial presentation.
Treatment
All cases received potassium gluconate (Tumil K powder, Daniels, Pharmaceuticals, St Louis, USA) (2–6 mmols bid PO) and spironolactone (H.N Norton and Co Ltd, Harlow, UK) (2.5 mg/kg sid PO). Cases 1–4, 6, 7, 12, and 13 also received amlodipine besylate (Istin, Pfizer Ltd Sandwich, UK) (0.125 mg/kg sid PO). Additionally cases 5–8 were initially given intravenous 0.9% sodium chloride infusions supplemented with 20–40 mmol/l of potassium chloride (Antigen Pharmaceuticals, Tipperary, Ireland) for 24 h to help correct the hypokalaemia. In none of the cases did medical treatment alone normalise potassium levels, although potassium concentrations did rise in all cases. However, all cases presenting with a myopathy did show resolution of these clinical signs with medical treatment despite the persistent hypokalaemia. All but two of the 11 hypertensive cases became normotensive with treatment. Cases 2 and 13 remained hypertensive (BPs of 200 and 220 mmHg, respectively) despite increasing the amlodipine dose to 0.125 mg/kg bid PO. Cases 1 and 2 showed no evidence of vision recovery. Case 13 was also treated with 5 mg tid PO carbimazole (Neomercazole, Roche Products Limited, Welwyn Garden City, UK) resulting in euthyroidism (thyroxine concentration of 35 nmol/l) 28 days later. Thereafter the carbimazole dose was reduced to 5 mg bid PO.
Cases 3–6 and 8–13 underwent abdominal laparotomy between days 13 and 183 after initial presentation. Cases 1, 2 and 7 remained on medical treatment only. Cases 4–6, 8, 12, and 13 underwent unilateral adrenalectomy without complication. Case 3 (Fig 3) had evidence of intra-abdominal haemorrhage 10 h after surgery necessitating repeat laparotomy. An autotransfusion was performed but no evidence of further haemorrhage was found at surgery and a body bandage was applied for the following 24 h. No further complications occurred. Case 9 was euthanased during surgery as abdominal laparotomy revealed adhesions between the liver and CVC and severe haemorrhage occurred when attempts to dissect the CVC were made. In cases 10 and 11 severe haemorrhage from the CVC occurred within an hour of surgery. Attempts to stop the bleeding failed at repeat laparotomy resulting in the decision to euthanase both cases (Table 2).
Fig 3.
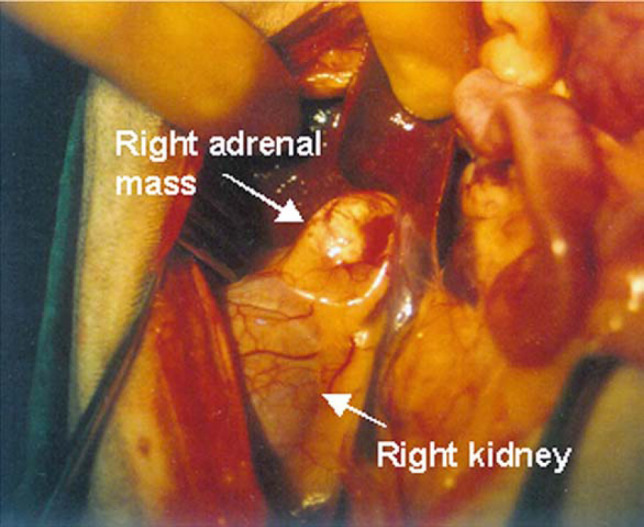
View of exploratory laparotomy in case 3 showing the cream coloured right adrenal mass closely adherent to the kidney. In man adrenal adenomas are said to be characterised by a golden yellow colour (Ganguly 1998).
Histopathology
Histopathology of adrenocortical tissue confirmed the presence of neoplasia in all 13 cases. Tissue was collected post mortem in cases 1, 2, and 7, and during surgical adrenalectomy in the remaining 10 cases. Of the latter 10 cases, five remain alive and five were euthanased. Of those euthanased after surgery, only cases 9 and 13 had post mortem examinations performed. Six cases had unilateral adrenal carcinomas. Adrenal adenomas were identified in seven cases; unilateral in five and bilateral in two. The bilateral nature of adrenal adenomas in cases 7 and 9 was only discovered at post mortem examination. No evidence of metastases was found in any case examined post mortem, but in all cases the adrenal mass was closely adherent to the CVC, and in case 9 to the liver also. In cases 1 and 2 bilateral renal interstitial nephritis was evident. In case 13 evidence of generalised sepsis was found in the liver, kidneys and lungs, and bilateral thyroid adenomas. Case 5 was euthanased because of a cranial abdominal mass but post mortem examination was not performed.
PAC measurements post-adrenalectomy
PACs were measured post-adrenalectomy in all six cases that survived at least 14 days post-adrenalectomy. In case 3 PAC was below the level of detection by the assay (<27.7 pmol/l) on day 50 post-adrenalectomy and PAC was normal (227 pmol/l) on day 1470. In case 4 PAC was normal when measured on days 170 (119.1 pmol/l), 350 (362.9 pmol/l) and 560 (116.3 pmol/l) post-adrenalectomy. In case 5 PAC was low (72 pmol/l) on day 40 post-adrenalectomy. In cases 6, 8 and 12 PAC was below the level of detection of the assay on either day 10 (for cases 6 and 8) or day 4 (for case 12) post-adrenalectomy.
Survival
Two of the three cases that were treated medically only were euthanased due to chronic renal failure (CRF) 304 and 984 days after presentation. The remaining cat was euthanased at 50 days with severe weakness due to the failure of the owner to medicate the cat beyond seven days. Of the 10 cases that underwent surgical adrenalectomy (following a successful stabilisation period of between 14 and 149 days on medical management) five are still alive at the time of writing with survival times of between 240 and 1803 days. The remaining five were either euthanased in the peri-operative period (cases 9, 10, and 11) because of surgical-induced haemorrhage, or euthanased because of septicaemia 14 days after surgery (case 13), or euthanased 1045 days after surgery due to anorexia and the presence of an enlarging cranial abdominal mass. In all cases that survived surgery medical management with amlodipine and spironolactone was stopped immediately following surgery, whilst potassium supplementation was gradually reduced over a period of between 1 and 6 days (Table 2).
Discussion
In man PHA is characterised by elevated PAC in the face of suppressed plasma renin activity (PRA) demonstrating renin–aldosterone dissociation, whereas SHA arises as a result of elevated PRA caused by cardiovascular failure, renal failure or severe hepatocellular dysfunction (Conn et al 1964). In order to confirm that the elevated PACs recorded in the current cases were caused by PHA, demonstration of suppressed PRA should ideally have been performed. However, PRA measurement was not possible in the current study as a validated assay for feline samples was not commercially available in the UK. Indeed another previously reported case of PHA in a cat in the UK (MacKay et al 1999) also failed to measure PRA and a diagnosis was based on elevated PAC in the presence of an adrenal tumour and appropriate clinical signs. Although PRA has been measured in five previously reported cases of feline PHA only two cases (Eger et al 1983, Moore et al 2000) actually had reduced PRA levels. In another two cases PRA was normal (Flood et al 1999), and in the one remaining case PRA was either normal or reduced when measured on two consecutive days (Rijnberk et al 2001b). These findings illustrate that even when PRA is measured, normal values have not ruled out the presence of hyperaldosteronism resulting from an adrenal tumour. The use of PRA per se in the diagnosis of PHA is, therefore, questionable. In man, similar problems arise and determining the ratio of PAC to PRA is believed to be more accurate in diagnosing cases of PHA (Ganguly 1998). To the authors' knowledge, the PAC to PRA ratio has not yet been used in the diagnosis of feline PHA cases, but may be a useful parameter to determine in future cases should a PRA assay become readily available.
All cases in the current series had the presence of an adrenocortical tumour confirmed on histopathology of tissues collected at either post mortem examination or surgery. Additionally, conditions associated with SHA in the cat are said to usually be associated with normokalaemia and the development of oedema (Ahn 1994). None of the current cases had oedema evident and all were hypokalaemic. Cases 7 and 10 had raised urea and creatinine concentrations at presentation, possibly suggesting renal disease, but their urine SGs were 1.038 and 1.040, respectively, showing reasonable renal concentrating ability. It is possible that renal disease could have contributed to the raised PACs of 8005 and 6230 pmol/l demonstrated in cases 7 and 10, but these PAC values are more elevated than those previously reported in cats with CRF (mean PAC of 499 pmol/l in a study by Jensen et al (1997) and 2656 pmol/l in a study by Mishina et al (1998)).
The authors believe that in the current case series the presence of adrenocortical neoplasia, hypokalaemia and/or hypertension, and markedly elevated PACs confirm the presence of PHA in these cases despite the failure to measure PRA.
In man numerous forms of hyperaldosteronism have been identified; aldosterone-producing adrenal adenomas and carcinomas, bilateral adrenal hyperplasia, primary (unilateral) adrenal hyperplasia, aldosterone-producing ovarian tumours, familial hyperaldosteronism types I and II and idiopathic hyperaldosteronism (Stewart 1999, Wheeler and Harris 2003). Histological examination of adrenocortical tissue in the cases in the current report revealed six carcinoma and seven adenoma (two bilateral and five unilateral) cases. Previous reports of histological examination of adrenocortical tissues in cats with PHA have been limited, and have all described initial unilateral disease. Although one adenoma (MacKay et al 1999) and one carcinoma (Eger et al 1983) have been described, other reports have not determined the precise nature of adrenal neoplasia using histopathology (Flood et al 1999, Moore et al 2000, Rijnberk et al 2001b). Our data suggest that adrenal carcinomas are quite common in the cat, contrary to the situation in man (Wheeler and Harris 2003). The case reported by MacKay et al (1999) was interesting in that a left adrenal adenoma was surgically removed but 2 years later the cat developed a right adrenal carcinoma. The finding of bilateral adrenal adenomas in two cases in the current series is the first time, to the authors' knowledge, that bilateral adenomas have been reported as a cause of PHA in the cat. The fact that the bilateral nature of adrenal disease in these two cases was only discovered post mortem suggests that clinicians should be aware of the possibility of bilateral disease in cases of feline PHA, and that both adrenals should be evaluated by imaging pre-operatively and at surgery if undertaken. Bilateral adrenal hyperplasia should be considered as a differential diagnosis for cases with documented bilateral adrenal enlargement although in the current series adenomatous change was confirmed on histopathology.
Ultrasonography or MRI of the adrenal glands proved useful as an aid to diagnosis in the cases described here, allowing visualisation of adrenal masses, although the bilateral nature of disease in case 9 was not seen on ultrasonography. In cases 9, 10 and 11 that died because of haemorrhage in the peri-operative period, close association with the CVC had been detected on ultrasonography in cases 10 and 11. However, an MRI in case 10 had showed no evidence of extension of the mass into the CVC. Pre-operative assessment of the likely ease of surgical removal of adrenal masses therefore appears to be problematic. Saphenous venography has been used in one feline case of PAH to demonstrate lack of invasion of the CVC by an adrenal tumour (Moore et al 2000) but subsequent surgical removal was not attempted in this case, thus the accuracy of saphenous venography at detecting CVC invasion was not determined. Computed tomography is the imaging modality of choice in man for evaluation of adrenal pathology in cases of hyperaldosteronism (Young 2003) and has been used successfully in both a feline (Rijnberk, et al 2001b) and canine (Rijnberk, et al 2001a) PHA case.
Interestingly, the presence of an adrenal carcinoma did not appear to be associated with a poorer prognosis than an adrenal adenoma. Only one of the three cases that were euthanased because of peri-operative haemorrhage associated with surgery was an adrenal carcinoma, and case 5 survived 1045 days following adrenalectomy of a carcinoma. In contrast the prognosis for adrenal carcinomas in man is much poorer than for adrenal adenomas (Wheeler and Harris 2003).
Clinical signs associated with hypokalaemic polymyopathy were commonly reported in this current series of PHA cases, being seen in 11 of the 13 cases, and were typical of those previously reported with hypokalaemia (Dow et al 1987). Fundic changes consistent with systemic hypertension were seen in five cases although only two of these cases showed vision deficits. Clinical signs of polymyopathy appeared to respond well to medical treatment with potassium supplementation and spironolactone, although serum potassium concentrations remained below the reference range, as has been reported previously (Rijnberk et al 2001b). Polyuria and polydipsia were reported in three cases. In case 13 polyuria and polydipsia may have arisen because of concurrent hyperthyroidism, but in cases 9 and 11 this may have been due to vasopressin resistance and increased osmotic threshold of vasopressin release, as has been documented in a canine case of PHA with polyuria and polydipsia (Rijnberk et al 2001a).
Hypernatraemia has been reported previously in two feline hyperaldosterone cases (Flood et al 1999, MacKay et al 1999) but was mild in both cases. The lack of significant hypernatraemia in the current cases may be explained by concurrent volume expansion reducing the effect of sodium retention on serum sodium concentrations, although total body sodium may still be elevated. In man PHA can be accompanied by serum sodium concentrations with the normal reference range (Ganguly 1998).
The authors suggest that PHA should no longer be considered a rare condition in cats. PAC should be measured and imaging of both the adrenal glands performed in all cases of apparently idiopathic feline hypertension and in cases presenting with hypokalaemia of unknown aetiology. Periods of remission of nearly five years can be obtained following adrenalectomy but this procedure carries with it a significant risk of severe haemorrhage. Medical therapy with potassium supplementation and spironolactone can be successful in ameliorating clinical signs of polymyopathy. Treatment with amlodipine can be used to manage hypertension but in some cases the hypertension is refractory to treatment.
Acknowledgements
Andrea Harvey holds a position funded by the Feline Advisory Bureau. The authors wish to thank their colleagues at Grove Lodge Veterinary Hospital and Bristol Veterinary School for their help in the management of these cases. Nicola Bromley, Martha Cannon, Sandy Enzerink, Michelle Flemming, Peter Fry, Alex Gale, Jim Hughes, Nicola Peters and Abingdon Vet Centre, London are thanked for referring cases. Ian Battersby and Kate Murphy are thanked for managing two of the cases at Bristol Veterinary School. Ralph Abercromy, Richard Coe, Eamon Draper, Peter Holt, Alasdair Hotston Moore and Emma Love are thanked for their input in performing the adrenalectomies and Jo Arthur, Sarah Band, Frances Barr, Kate Bradley, Margaret Costello and Ruth Dennis for imaging expertise. Histopathological examination of the University of Bristol cases was performed by Michael Day, Janet Bradshaw and Geoff Pearson of the Division of Veterinary Pathology, Infection and Immunity, University of Bristol.
References
- Ahn A. Hyperaldosteronism in cats, Seminars in Veterinary Medicine and Surgery (Small Animal) 9, 1994, 153–157. [PubMed] [Google Scholar]
- Birchard S.J. Adrenalectomy. Slatter D. Textbook of Small Animal Surgery, 1993, W.B. Saunders: Philadelphia, PA, 1510–1518. [Google Scholar]
- Breitschwerdt E.B., Meuten D.J., Greenfield C.L., Anson L.W., Cook C.S., Fulghum R.E. Idiopathic hyperaldosteronism in a dog, Journal of the American Veterinary Medical Association 187, 1985, 841–845. [PubMed] [Google Scholar]
- Conn J.W. Primary aldosteronism, Journal of Laboratory Clinical Medicine 45, 1955, 661–664. [PubMed] [Google Scholar]
- Conn J.W., Cohen E.L., Rovner D.R. Suppression of plasma renin activity in primary aldosteronism, Journal of the American Medical Association 190, 1964, 213–221. [DOI] [PubMed] [Google Scholar]
- Dow S.W., LeCouteur R.A., Fettman M.J., Spurgeon T.L. Potassium depletion in cats: hypokalemic polymyopathy, Journal of the American Veterinary Medical Association 191, 1987, 1563–1568. [PubMed] [Google Scholar]
- Eger C., Robinson W., Huxtable C. Primary aldosteronism (Conn's syndrome) in a cat; a case report and review of comparative aspects, Journal of Small Animal Practice 24, 1983, 293–307. [Google Scholar]
- Feldman E.C., Nelson R.W. Canine and Feline Endocrinology and Reproduction, 2004, W.B. Saunders: Philadelphia, PA. [Google Scholar]
- Flood S.M., Randolph J.F., Gelzer A.R., Refsal K. Primary hyperaldosteronism in two cats, Journal of the American Animal Hospital Association 35, 1999, 411–416. [DOI] [PubMed] [Google Scholar]
- Ganguly A. Primary aldosteronism, New England Journal of Medicine 339, 1998, 1828–1834. [DOI] [PubMed] [Google Scholar]
- Jensen J., Henik R.A., Brownfield M., Armstrong J. Plasma renin activity and angiotensin I and aldosterone concentrations in cats with hypertension associated with chronic renal disease, American Journal of Veterinary Research 58, 1997, 535–540. [PubMed] [Google Scholar]
- MacKay A.D., Holt P.E., Sparkes A.H. Successful surgical treatment of a cat with primary aldosteronism, Journal of Feline Medicine and Surgery 1, 1999, 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M., Watanabe T., Fujii K., Maeda H., Wakao Y., Takahashi M. Non-invasive blood pressure measurements in cats: clinical significance of hypertension associated with chronic renal failure, Journal of Veterinary Medical Science 60, 1998, 805–808. [DOI] [PubMed] [Google Scholar]
- Moore L.E., Biller D.S., Smith T.A. Use of abdominal ultrasonography in the diagnosis of primary hyperaldosteronism in a cat, Journal of the American Veterinary Medical Association 217, 2000, 213–215. [DOI] [PubMed] [Google Scholar]
- Rijnberk A., Kooistra H.S., van Vonderen I.K., Mol J.A., Voorhout G., van Sluijs F.J., van den Ingh T.S., Boer P., Boer W.H. Aldosteronoma in a dog with polyuria as the leading symptom, Domestic Animal Endocrinology 20, 2001a, 227–240. [DOI] [PubMed] [Google Scholar]
- Rijnberk A., Voorhout G., Kooistra H.S., van der Waarden R.J., van Sluijs F.J., Boer P., Boer W.H. Hyperaldosteronism in a cat with metastasised adrenocortical tumour, Veterinary Quarterly 23, 2001b, 38–43. [DOI] [PubMed] [Google Scholar]
- Sparkes A.H., Caney S.M., King M.C., Gruffydd-Jones T.J. Inter- and intraindividual variation in Doppler ultrasonic indirect blood pressure measurements in healthy cats, Journal of Veterinary Internal Medicine 13, 1999, 314–318. [DOI] [PubMed] [Google Scholar]
- Stewart P.M. Mineralocorticoid hypertension, Lancet 353, 1999, 1341–1347. [DOI] [PubMed] [Google Scholar]
- Wheeler M.H., Harris D.A. Diagnosis and management of primary aldosteronism, World Journal of Surgery 27, 2003, 627–631. [DOI] [PubMed] [Google Scholar]
- Young W.F., Jr. Minireview: primary aldosteronism—changing concepts in diagnosis and treatment, Endocrinology 144, 2003, 2208–2213. [DOI] [PubMed] [Google Scholar]