Abstract
The prevalence of urinary tract infections (UTIs) in cats with hyperthyroidism (n=90), diabetes mellitus (DM) (n=57) and chronic kidney disease (CKD) (n=77) was evaluated retrospectively. It was found to be 12% in cats with hyperthyroidism and DM, respectively, and 22% in cats with CKD. Associations between UTIs and clinical signs, biochemical markers in serum and urinalyses were investigated. Many of the cats with UTIs had no clinical signs of lower urinary tract disease or changes in their laboratory values indicative of infection. Therefore, a urinalysis alone should not be used to exclude UTIs in these cats. UTIs are relatively common in cats with hyperthyroidism, DM and CKD, and urine cultures are recommended as part of the basic diagnostic plan for cats suspected of suffering from these conditions.
Hyperthyroidism is a very common endocrine disease in older cats, affecting approximately 1/300 cats, and has been diagnosed with increasing incidence since the 1980s (Gerber et al 1994; Edinboro et al 2004). Common thyrotoxic complications are hypertension, heart failure and chronic kidney disease (CKD) (Gunn-Moore 2005). To the authors' knowledge urinary tract infections (UTIs) have not been reported to be common in cats or humans with hyperthyroidism.
Multiple studies have been published on humans with diabetes mellitus (DM) reporting a high prevalence of bacteriuria and an increased risk for pyelonephritis (Geerlings et al 2000, 2001; Ronald and Ludwig 2001; Stapleton 2002). Various publications also showed a high prevalence of UTIs in humans with CKD, with asymptomatic bacteriuria being a common finding in these patients (Saitoh et al 1985; Rai et al 2002). In one study the incidence of bacterial cystitis in 158 dogs and 71 cats with DM was 12.7% in dogs and 9.9% in cats, and females were found to have a higher prevalence of UTIs when compared to males (Kirsch 1998). In another study the incidence of UTIs was reported to be 24% in diabetic dogs, with the infected dogs having an increased incidence of bacteria in their urine sediment, and a greater percentage of females being present in the positive culture group (McGuire et al 2002). A retrospective study describing the clinico-pathological findings associated with CKD in cats showed a prevalence of bacterial UTI of 16.7% (DiBartola et al 1987). Recently, Bailiff presented the preliminary results of a study assessing the prevalence of UTIs in 879 cats seen at a university teaching hospital. The overall prevalence of UTIs was 15%; cats with DM had a prevalence of 13%, cats with CKD 18%, and cats with miscellaneous diseases had a 16% prevalence of UTIs. Of the miscellaneous group a small subdivision of cats with hyperthyroidism had a prevalence of UTIs of 24% (Bailiff et al 2004). However, many of the cats were suffering from multiple conditions making conclusions about specific diseases and their association with UTIs difficult. There was also no attempt to correlate clinico-pathological data with the occurrence of UTIs in these cats.
The goals of this study were to evaluate the prevalence of UTIs in cats with three common diseases: hyperthyroidism, CKD and DM, and to identify associations between the clinical presentation, biochemical data, urinalyses and urine culture results in those cats.
Materials and methods
Case selection
Medical records of cats with a diagnosis of hyperthyroidism, DM and CKD presenting to Cornell University Hospital for Animals (CUHA) from January 1996 through December 2003 were reviewed. Cats were enrolled if their medical record documented a urine culture with urine collected by cystocentesis or catheterization, a urinalysis and non-equivocal evidence of the primary diagnosis. At CUHA a urine culture was considered part of the basic diagnostic work-up in cats presenting with these diseases during the years of the study. Cats were excluded if there was evidence in the record of urethral catheterization or treatment with antibiotics in the month before presentation. Cats with concurrent disorders were excluded from the hyperthyroid group, but not from the DM or CKD group if the concurrent disorder was not thought to have a major impact on the lower urinary tract. This is because the vast majority of cats with DM and some of the cats with CKD suffered from concurrent disorders. Intact and neutered cats were not separated for data analysis.
Hyperthyroid cats
The diagnosis of hyperthyroidism was based on appropriate history, clinical findings and laboratory data including a complete blood count (CBC), serum chemistry panel, increased (at presentation or historical) total serum thyroxin concentration (T4) (reference range: 1.5–4.0 μg/dl, 19.4–51.6 nmol/l) or free serum thyroxin concentration (fT4) (reference range 1.5–4 ng/dl, 19.4–51.6 pmol/l) and a positive thyroid scintigraphy scan with pertechnetate. Methimazole treatment was completed at least 1 week before their presentation. Individual cases were further evaluated with electrocardiography, blood pressure measurements, thoracic radiographs, abdominal ultrasound and echocardiogram to rule out cardiac or renal disease. All cats included were treated with radioactive iodine. Post iodine treatment blood work included CBC, serum chemistry panel and total T4. Concurrent renal failure was ruled out to the best of the authors' ability and cats were included only if the serum chemistry obtained following radioactive iodine therapy demonstrated blood urea nitrogen and serum creatinine concentrations within the reference range.
Cats with DM
The diagnosis of DM was also based on history, clinical findings and laboratory data including a CBC, serum chemistry panel, and urinalysis. Hyperglycemia (>140 mg/dl, 7.8 mmol/l) and glucosuria, increased fructosamine concentration (>325 μmol/l) and/or treatment with insulin had to be documented in the record for inclusion.
Cats with CKD
The diagnosis of CKD was based on history, clinical signs and laboratory data (CBC, serum chemistry panel, and urinalysis). All cats included in the study had to have increased blood urea nitrogen (>35 mg/dl, 12.5 mmol/l) and creatinine concentrations (>2.1 mg/dl, 186 μmol/l) and a low urine specific gravity (USG) (<1.030). These values had to be documented at least 4 weeks prior to and again during the visit when the urine culture was obtained.
Laboratory data
CBCs, serum chemistry panels and urinalyses were performed at Cornell University in a standard fashion. USG was determined by refractometer, urine glucose, ketones and bilirubin concentration was measured using a dipstick. Urine protein concentration was determined by the sulfosalycilic acid (SSA) method. Urine sediment was examined microscopically to evaluate for the presence of white blood cells (WBCs), bacteria, epithelial cells, casts and crystals. Trained laboratory technicians performed urine sediment examinations. Hematuria and urine red blood cells (RBCs) were not included in this study due to urine collection by cystocentesis. Quantitative urine cultures were performed in all cases. Reported bacterial growth was considered a UTI. Commercial plates (Sensitire veterinary urinary two isolate MIC, Trek Diagnostic System) were used for standardized urine minimum inhibitory concentration (MIC) testing for ampicillin, amoxycillin/clavulanic acid, ceftiofur, cephalexin, enrofloxacin, tetracycline and trimethoprim/sulfonamide (TMS). Microscopic evidence of bacteria on evaluation of the urine sediment was considered bacteriuria and a positive urine culture was considered a UTI.
Statistical analysis
The prevalence of UTIs in each group and the confidence interval (CI 95%) was calculated. The χ2 test was used to look for an association between clinical signs of lower urinary tract disease (LUTD) and positive cultures. Fisher's exact test was used to find associations between clinical signs and biochemical markers in blood tests and urinalysis and UTIs in each group separately. Fisher's exact test was also used to look for interrelations when associated factors were found. A one-sided approach was chosen for all questions. All data were divided into two groups, male and female, treatment and no treatment, physiological and pathological, values in reference range or above. Intact and neutered cats were not separated for the purpose of analysis. The cut off for defining low USG was chosen by the authors at 1.020, and for high urine pH at 7.5, because low USG and alkaline pH have been associated with a higher risk for bacterial growth (Lees et al 1979). A P-value of less than 0.05 was considered significant. All statistical analyses were performed using commercially available software (Statistix 8; Analytical Software, Tallahassee, FL 32317-2185).
Results
Two hundred and twenty-four cats were included in the study: 90 with hyperthyroidism, 57 with DM, and 77 with CKD (Table 1, Figs 1–5).
Table 1.
Signalment, history and clinico-pathological data from 224 cats
| Hyperthyroidism | Diabetes mellitus | Chronic kidney disease | ||||
|---|---|---|---|---|---|---|
| All | With UTI | All | With UTI | All | With UTI | |
| Total number | 90 | 11 | 57 | 7 | 77 | 17 |
| Female | 52 | 10 | 21 | 3 | 39 | 14 |
| Male | 38 | 1 | 36 | 4 | 38 | 3 |
| Age range (in years) | 4–18 | 9–18 | 1–18 | 9–18 | 2–19 | 2–18 |
| Age median (in years) | 13.0 | 14.0 | 12.0 | 12.5 | 13.0 | 13.5 |
| History | ||||||
| Polyuria/polydipsia | 11 | 2 | 30 | 1 | 29 | 5 |
| Signs of LUTD | 2 | 0 | 3 | 2 | 6 | 4 |
| Other health problems | 0 | 0 | 23 | 0 | 11 | 4 |
| Treated with methimazole | 42 | 6 | 0 | 0 | 0 | 0 |
| Treatment with steroids | 0 | 0 | 6 | 0 | 0 | 0 |
| Treatment with insulin | 0 | 0 | 34 | 5 | 0 | 0 |
| Complete blood count | ||||||
| Hematocrit decreased | 3 | 1 | 2 | 0 | 25 | 6 |
| Hematocrit increased | 3 | 1 | 1 | 0 | 0 | 0 |
| WBC increased | 10 | 2 | 29 | 1 | 19 | 4 |
| Left shift | 1 | 0 | 14 | 3 | 10 | 2 |
| Serum chemistry | ||||||
| Glucose 1 >140 mg/dl (7.8 mmol/l) | 10 | 1 | 50 | 3 | 16 | 5 |
| Glucose 1 >250 mg/dl (13.9 mmol/l) | 0 | 0 | 42 | 2 | 0 | 0 |
| Urea nitrogen 2 increased | 6 | 0 | 15 | 4 | 77 | 16 |
| Creatinine 3 increased | 0 | 0 | 2 | 1 | 77 | 17 |
| tT4 * 4 measured | 83 | 9 | 31 | 2 | 31 | 6 |
| tT4 * 4 increased | 78 | 9 | 0 | 0 | 0 | 0 |
| tT4 * 4 decreased | 0 | 0 | 23 | 2 | 18 | 3 |
| Mean tT4 * 4 in μg/dl (mmol/l) | 11.6 (150) | 13.4 (173) | 1.1 (14) | 0.6 (8) | 1.4 (18) | 1.4 (18) |
| Urinalysis | ||||||
| Gross appearance abnormal 5 | 27 | 5 | 7 | 1 | 3 | 1 |
| Urine specific gravity >1.020 | 82 | 11 | 47 | 2 | 5 | 2 |
| Urine specific gravity mean | 1.029 | 1.036 | 1.034 | 1.019 | 1.013 | 1.012 |
| Urine dipstick | ||||||
| Glucosuria | 6 (mild) | 1 | 52 | 3 | 4 (mild) | 2 |
| Ketonuria | 3 (trace) | 1 | 10 | 1 | 0 | 1 |
| Bilirubinuria | 0 | 0 | 5 | 1 | 0 | 0 |
| pH ≥7.5 | 10 | 2 | 3 | 1 | 5 | 0 |
| Proteinuria ≥1+ | 29 | 4 | 16 | 2 | 24 | 9 |
| Urine sediment | ||||||
| WBC >5/HPF | 4 | 2 | 4 | 2 | 13 | 11 |
| Bacteria seen | 14 | 8 | 6 | 5 | 14 | 14 |
| Casts seen | 6 | 0 | 5 | 2 | 6 | 1 |
| Crystals seen | 15 | 0 | 10 | 2 | 7 | 2 |
| Urine collection | ||||||
| Cystocentesis | 90 | 11 | 54 | 5 | 77 | 17 |
| Catheterization | 0 | 0 | 3 | 2 | 0 | 0 |
=Total T4, UTI=urinary tract infection, LUTD=lower urinary tract disease, WBC=white blood cell, HPF=high-power field. Reference ranges:
serum glucose 63–140 mg/dl (3.5–7.8 mmol/l);
serum urea nitrogen 17–35 mg/dl (6.1–12.5 mmol/l);
serum creatinine 0.7–2.1 mg/dl (62–186 μmol/l);
total T4 1.5–4.0 μg/dl (19.4–51.6 nmol/l);
abnormal urine color and turbidity: orange, brown or red and cloudy or opaque.
Fig 1.
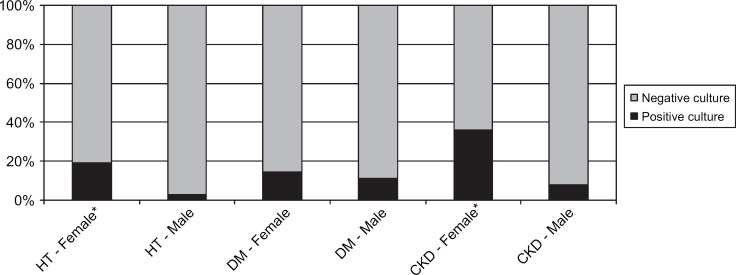
Gender. HT=hyperthyroidism, DM=diabetes mellitus, CKD=chronic kidney disease.
Fig 2.
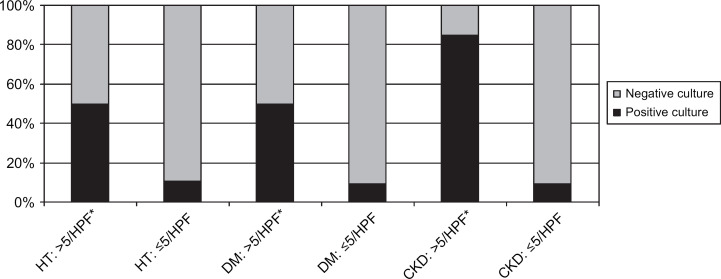
WBC seen in urine sediment. HPF=high-power field.
Fig 3.
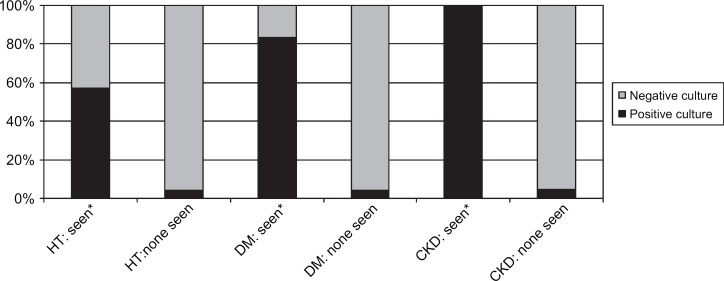
Bacteria seen in urine sediment. HT=hyperthyroidism, DM=diabetes mellitus, CKD=chronic kidney disease.
Fig 4.
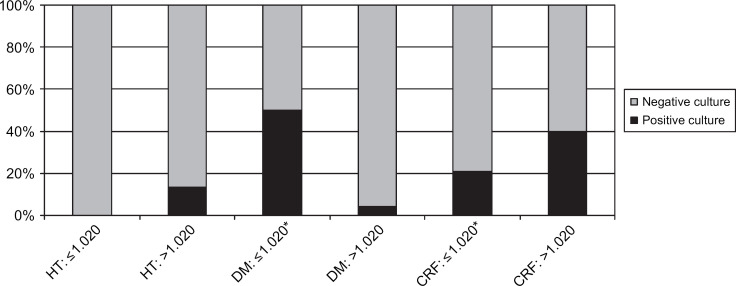
Urine specific gravity (USG). HT=hyperthyroidism, DM=diabetes mellitus, CKD=chronic kidney disease.
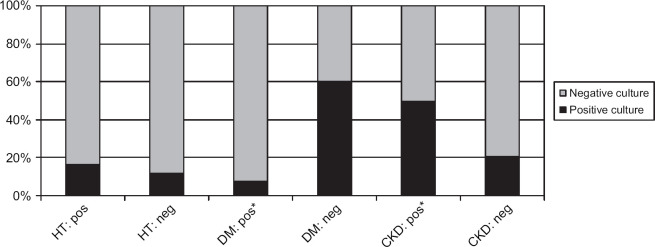
Fig 5.
Glucosuria (dipstick). *Significant association with positive urine culture. HT=hyperthyroidism, DM=diabetes mellitus, CKD=chronic kidney disease.
Hyperthyroidism
In the group of cats with hyperthyroidism two were reported as having signs of LUTD; one with pollakiuria, one with hematuria. Twelve percent of cats had a positive urine culture (11/90: CI 5–20%). None had clinical signs of LUTD reported in their record, or abnormalities in the CBCs or serum chemistry panels. An association between UTIs and female gender (P=0.006) was identified. No association was found with age greater than 10 years. An association was found between UTIs and bacteriuria (P<0.0001), as well as increased WBC in the urine sediment (P<0.0001). These two variables were interrelated (P<0.0001). No associations were found with variables of the CBC and other variables of the urinalysis. No additional associations were found when including only cats with a markedly increased total serum T4 concentration (tT4>7.0 μg/dl) or only cats without prior methimazole therapy.
Diabetes mellitus
In the cats with DM concurrent disorders included feline asthma (n=4), hypertrophic cardiomyopathy (n=3), corneal ulcer (n=2), hepatic lipidosis (n=3), pancreatitis (n=5), cholangiohepatitis (n=4), gastroenteritis or inflammatory bowel disease (n=6). These cats had also been treated historically or concurrently with glucocorticoids, or for eosinophilic granuloma (n=1), seizures (n=2), chronic respiratory infections (n=1), stomatitis (n=1) and abscess (n=1). Blood work was performed at the time of the urine culture in 53 cats. The remaining four had blood work performed at CUHA at other presentations within the same year. Three cats had a blood glucose concentration within the reference range and five had no glucosuria. All had been treated with insulin.
The prevalence of UTIs in cats with DM was 12% (7/57; CI 3–22%). One cat was reported to show dysuria and stranguria. No associations were identified between UTIs and female gender or UTIs and age >10 years. An association was found between UTIs and bacteriuria (P<0.0001) and increased WBC (P<0.0001). These two variables were interrelated (P=0.05). Associations were also found between UTIs and low USG (USG<1.020) and glucosuria (P=0.01). No associations were found with variables of the CBC and other variables of the urinalysis.
Chronic kidney disease
Concurrent diseases identified in cats with CKD were heart disease (n=3), inflammatory bowel disease (n=3), fever (n=2) and seizures (n=1). Fifty-six cats had ultrasonographic findings consistent with CKD; reduction in size and abnormalities in shape and architecture of the kidneys were reported. The rest of the cats did not have abdominal ultrasound performed at CUHA. Serum creatinine concentrations were measured at the same time as urinalysis and urine culture in 76 cats, the remaining cat had blood work performed at another visit within the same year. The mean serum creatinine concentration was 4.3 mg/dl (range 2.2–19.2 mg/dl).
Positive urine cultures were found in 22% of cats with CKD (17/77; CI 12–32%). Four cats were found to have signs of LUTD. An association between UTIs and female gender (P=0.002) was identified. No association was found between UTIs and age >10 years. An association was present between UTIs and bacteriuria (P<0.0001) and increased WBC seen in the urine sediment (P<0.0001). These two variables were interrelated (P<0.0001). An association was also present between UTIs and glucosuria (P<0.0001). No associations were found with variables of the CBC and other variables of the urinalysis. Furthermore, no association was found between UTIs and markedly increased serum creatinine concentrations (>5.0 mg/dl).
Associations between urine cultures and clinical signs
All cats with hyperthyroidism, DM and CKD were combined to look for associations between UTIs and clinical signs of LUTD. Thirty-five of 224 cats had UTIs. Only 11 of the 224 cats had signs of pollakiuria, stranguria, dysuria and/or hematuria reported, six of these had UTIs. An association between UTIs and clinical signs of LUTD was identified (P=0.005).
Urine cultures and susceptibility
The results of the 35 positive urine cultures are presented in Table 2. Thirty-two bacterial isolates (89%) were sensitive to amoxycillin/clavulanic acid with only two cases of Streptoccocus species group D and two of Pseudomonas aeroginosa being resistant at tested urine MIC. The only resistance to enrofloxacin was reported in one case of Streptococcus species group D. Resistance was frequent to ceftiofur and cephalexin, especially with the genus Enterobacter faecalis. This genus also showed in vitro intermediate sensitivities to enrofloxacin in five cases. Pseudomonas aeroginosa were highly resistant in both cases with reported sensitivities to enrofloxacin.
Table 2.
Results of 35 positive urine cultures
| Total number | Hyperthyroidism | Diabetes mellitus | CKD | |
|---|---|---|---|---|
| 35 | 11 | 7 | 17 | |
| Genera of bacteria | 37 | 12 | 8 | 17 |
| Escherichia coli | 17 (46.0%) | 4 | 3 | 10 |
| Enterococcus faecalis | 10 (27.0%) | 4 | 4 | 2 |
| Staphylococcus species | 2 (5.4%) | 2 | ||
| Streptococcus species | 2 (5.4%) | 1 | 1 | |
| Pseudomonas aeroginosa | 2 (5.4%) | 2 | ||
| Enterobacter species | 1 (2.7%) | 1 | ||
| Pasteurella multocida | 1 (2.7%) | 1 | ||
| Proteus mirabilis | 1 (2.7%) | 1 | ||
| Citrobacter freundii | 1 (2.7%) | 1 |
CKD=chronic kidney disease.
Discussion
A prevalence of bacterial UTI of 12% was identified in hyperthyroid cats. However, urinalyses did not provide any common specific risk factors for UTIs in this group. Glucosuria and low specific gravity were a rare finding and when they occurred they did not appear to be associated with a UTI. Mild proteinuria was found in one-third of the hyperthyroid cats. This may be attributed to hypertension and increased glomerular filtration rate (GFR) or glomerular pressure. Mild pre-glomerular proteinuria has been reported in the majority of thyrotoxic human and feline patients because of increased GFR, especially if intrarenal or systemic hypertension is present (Ford et al 1989; Gunn-Moore 2005; Langston and Reine 2006). Systemic blood pressure and urine protein:creatinine ratios were not assessed in most cats in this study; therefore further conclusions could not be drawn. No association was found between proteinuria and positive urine cultures in this group of cats. As the mean USG was 1.029 in this group, compromised renal function could not be completely ruled out as a factor in the pathogenesis of UTIs, though the mean USG of cats with UTIs was 1.036 and no association was found between a low USG and UTIs. Experimentally, thyroid hormones have been shown to modulate the immune response, but hyperthyroidism has not been associated with immune compromise or increased likelihood of infection in humans and rats (Botella-Carretero et al 2005; Johnson et al 2005; Vinayagamoorthi et al 2005; Klecha et al 2006). Further studies are necessary to evaluate renal and immune system function of hyperthyroid cats compared to healthy older cats to explain this apparent increased risk for UTIs.
An increased prevalence of UTIs has previously been suspected in cats with DM and CKD (Saitoh et al 1985; DiBartola et al 1987; Kirsch 1998; Geerlings et al 2000; McGuire et al 2002; Rai et al 2002). Low USG and glucosuria have been suggested to predispose diabetics to bacterial UTIs (Lees et al 1979; Lees 1996). Our data showed significant associations between UTIs and both factors in diabetic cats. Other factors that have been suggested to play a role in the pathogenesis of UTIs in humans and animals with DM are diabetic microangiopathy and impaired leukocyte function (Rayfield et al 1982; Forrester 1999; Stapleton 2002). Cats with CKD showed a positive association between UTIs and glucosuria. Increased blood glucose concentration above the renal threshold for glucose reabsorption could theoretically be the cause for the glucosuria in these cats. However, none of the cats in this group had an increase in blood glucose of greater than 250 mg/dl (13.75 mmol/l), the value that is thought to be consistent with the renal threshold in cats (Kruth and Cowgill 1982). Therefore, renal tubular damage may be the cause of glucosuria in these cats. It is unclear whether the association between glucosuria and increased risk for UTI is a result of the cats having a potential proximal tubular disorder or the possibility that glucose, a good substrate for bacterial growth in the urinary bladder, predisposes to UTI in cats with CKD regardless of the type of renal injury.
The DM group included cats with concurrent diseases including six with historical administration of glucocorticoid therapy. Excluding them would have severely limited our ability to assess UTIs in cats with DM. Their inclusion could have affected the prevalence of UTIs in this group but appeared not to, as none of these cats had UTIs at the time of urine culture.
No associations were found between increased blood leukocyte counts and UTIs. This is consistent with the notion that lower UTIs typically do not influence the results of routine blood tests (Osborne and Lees, 1995; Bartges 2004).
Urinalyses were shown to be a useful predictor for UTIs; there were associations between positive urine cultures and bacteriuria and increased WBCs in the urine sediment in all three groups. Nevertheless, eight cats with UTIs had no bacteriuria and six of these had no abnormal findings in their urine sediment. Therefore, urine cultures were often necessary to diagnose UTIs in cats with these diseases, reinforcing the claim that quantitative urine culture is the gold standard for identifying bacterial UTIs (Bartges 2004).
The data in this study documented an association between UTIs and signs of LUTD in cats with hyperthyroidism, DM and CKD. In older cats these signs are more likely to be associated with bacterial infection compared to young cats with the same presentation. It remains unclear whether this is due to age, underlying disease or a combination of the two (Bartges 2003). However, of the 35 cats with UTI only six showed clinical signs of LUTD. More research in geriatric cats without evidence of underlying disease is necessary to come to clear conclusions in this matter. In the mean time it is reasonable to recommend searching for underlying endocrine or metabolic diseases in older cats presenting with lower urinary signs and/or UTIs.
Significant associations between the female gender and UTIs in cats with hyperthyroidism and CKD were found. It is unclear why this could not be shown for cats with DM. Other studies in humans and in animals have shown a greater prevalence of UTIs in females with DM (Patterson and Andriole 1995; Kirsch 1998; Forrester 1999; Ronald and Ludwig 2001; Stapleton 2002). A shorter and wider urethra has been proposed to be the reason for a higher risk of UTIs in females (Osborne et al 1979; Kirsch 1998).
Urine culture results in this study are comparable to previous studies. Infections caused by Escherichia coli have been reported to account for one-third to one-half of all organisms isolated from the urine of infected cats (Lees 1996). An additional study reported that bacteria belonging to nine different genera cause nearly 99% of UTI in cats; Escherichia coli accounted for 52% of the infections (Davidson et al 1992). In our study there were no major differences in the pattern of distribution of the different genera of bacteria in cats with hyperthyroidism, DM and CKD.
Of cultured bacteria 89% of the isolates were sensitive to amoxycillin/clavulanic acid and 83% to enrofloxacin. Considering these results amoxycillin/clavulanic acid seemed to be a good first choice antibiotic. Nevertheless a few very resistant genera of bacteria were also found. Both the human and veterinary literature recommend antibacterial susceptibility testing to choose an appropriate antibiotic therapy (Bartges 2004; Wilson and Gaido 2004). This seemed especially important in patients with underlying metabolic diseases, immune compromise, changes in urine consistencies and therefore greater risk for persistent bacterial UTIs or pyelonephritis (Stamm and Hooton 1993; Senior 2000).
In summary, this study showed a high prevalence of bacterial UTI in cats with hyperthyroidism (12%), DM (12%) and CKD (22%). Associations were found between positive urine cultures and clinical signs of LUTD, and between positive urine cultures, bacteria and leukocytes in the urine sediment. Nevertheless, many of the cats with positive urine cultures had no clinical signs of LUTD, changes in blood work, or urinalysis indicating infection. Therefore, a urinalysis alone should not be used to exclude UTIs in these cats and urine cultures are recommended. Antibacterial susceptibility testing should follow every positive urine culture. Of the antibiotics tested, amoxycillin/clavulanic acid seemed to be a good initial choice for the treatment of most Gram-negative and Gram-positive bacteria.
References
- Bailiff N, Nelson R, Jang S, Westropp J. (2004) Prevalence or urinary tract infections in diabetic cats. In: Congress Proceedings 22nd ACVIM Forum, Minneapolis, MN, Abstract 218. p. 863.
- Bartges JW. (2003) Feline lower urinary tract cases. In: Congress Proceedings 21st ACVIM Forum, Charlotte, NC. pp. 579–581.
- Bartges J.W. Diagnosis of urinary tract infections, Veterinary Clinics of North America: Small Animal Practice 34, 2004, 923–933. [DOI] [PubMed] [Google Scholar]
- Botella-Carretero J.I., Prados A., Manzano L., Montero M.T., Sancho J., Escobar-Morreale H.F. The effects of thyroid hormones on circulating markers of cell-mediated immune response, as studied in patients with differentiated thyroid carcinoma before and during thyroxin withdrawal, European Journal of Endocrinology 153, 2005, 223–230. [DOI] [PubMed] [Google Scholar]
- Davidson A.P., Ling G.V., Stevens F., Franti C.E., Johnson D.L., Lang S.S. Urinary tract infections in cats: a retrospective study, 1977–1989, California Veterinarian 46 (5), 1992, 32–34. [Google Scholar]
- DiBartola S.P., Rutgers H.C., Zack P.M., Tarr M.J. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973–1984), Journal of the American Veterinary Medical Association 190, 1987, 1196–1202. [PubMed] [Google Scholar]
- Edinboro C.H., Scott-Moncrieff J.C., Janovitz E., Thacker L., Glickman L.T. Epidemiologic study of the relationships between consumption of commercial canned food and risk of hyperthyroidism in cats, Journal of the American Veterinary Medical Association 224, 2004, 879–886. [DOI] [PubMed] [Google Scholar]
- Ford H.C., Lim W.C., Chisnall W.N., Pearce J.M. Renal function and electrolyte levels in hyperthyroidism: urinary protein excretion and the plasma concentrations of urea, creatinine, uric acid, hydrogen ion and electrolytes, Clinical Endocrinology 30, 1989, 293–301. [DOI] [PubMed] [Google Scholar]
- Forrester S.D., Troy G.C., Dalton M.N., Huffman J.W., Holtzman G. Retrospective evaluation of urinary tract infections in 42 dogs with hyperadrenocorticism or diabetes mellitus or both, Journal of Veterinary Internal Medicine 13, 1999, 557–560. [DOI] [PubMed] [Google Scholar]
- Geerlings S.E., Stolk R.P., Camps M.J., Netten P.M., Hoekstra J.B.L., Bouter P.K., Bravenboer B., Collet T.J., Jansz A.R., Hoepelman A.I.M. Asymptomatic bacteriuria may be considered a complication in women with diabetes, Diabetes Care 23, 2000, 744–749. [DOI] [PubMed] [Google Scholar]
- Geerlings S.E., Stolk R.P., Camps M.J., Netten P.M., Collet T.J., Schneeberger P.M., Hoepelman A.I.M. Consequences of asymptomatic bacteriuria in women with diabetes mellitus, Archives of Internal Medicine 61, 2001, 1421–1427. [DOI] [PubMed] [Google Scholar]
- Gerber H., Peter H., Ferguson D.C., Peterson M.E. Etiopathology of feline toxic nodular goiter, Veterinary Clinics of North America: Small Animal Practice 24, 1994, 541–565. [DOI] [PubMed] [Google Scholar]
- Gunn-Moore D.A. Feline endocrinopathies, Veterinary Clinics of North America: Small Animal Practice 35, 2005, 171–210. [DOI] [PubMed] [Google Scholar]
- Johnson E.O., Kamilaris T.C., Calogero A.E., Gold P.W., Chrousos G.P. Experimentally-induced hyperthyroidism is associated with activation of the rat hypothalamic-pituitary-adrenal axis, European Journal of Endocrinology 153, 2005, 177–185. [DOI] [PubMed] [Google Scholar]
- Kirsch M. Incidence of bacterial cystitis in recently diagnosed diabetic dogs and cats. Retrospective study 1990–1996, Tierärztliche Praxis 26 (K), 1998, 32–36. [PubMed] [Google Scholar]
- Klecha A.J., Genaro A.M., Gorelik G., Arcos M.L. Barreiro, Silberman D. Magalí, Schuman M., Garcia S.I., Pirola C., Cremaschi G.A. Integrative study of hypothalamus–pituitary–thyroid-immune system interaction: thyroid hormone-mediated modulation of lymphocyte activity through protein kinase C pathway, Journal of Endocrinology 189, 2006, 45–55. [DOI] [PubMed] [Google Scholar]
- Kruth SA, Cowgill LD. (1982) Renal glucose transport in the cat. In: Congress Proceedings ACVIM, Washington, DC. p. 78.
- Langston C.E., Reine N.J. Hyperthyroidism and the kidney, Clinical Techniques in Small Animal Practice 21, 2006, 17–21. [DOI] [PubMed] [Google Scholar]
- Lees G.E. Bacterial urinary tract infections, Veterinary Clinics of North America: Small Animal Practice 26, 1996, 297–304. [PubMed] [Google Scholar]
- Lees G.E., Osborne C.A., Stevens J.B. Urine: a medium for bacterial growth, Veterinary Clinics of North America: Small Animal Practice 9, 1979, 611–616. [DOI] [PubMed] [Google Scholar]
- McGuire N.C., Schulman R., Ridgway M.D., Bollero G. Detection of occult urinary tract infections in dogs with diabetes mellitus, Journal of the American Animal Hospital Association 38, 2002, 541–544. [DOI] [PubMed] [Google Scholar]
- Osborne C.A., Klausner J.S., Lees G.E. Urinary tract infections: normal and abnormal host defence mechanisms, Veterinary Clinics of North America: Small Animal Practice 9, 1979, 587–609. [DOI] [PubMed] [Google Scholar]
- Osborne C.A., Lees G.E. Bacterial infections of the canine and feline urinary tract. Osborne C.A., Finco D.R. Canine and Feline Nephrology and Urology, 1995, Williams & Wilkins: Baltimore, 759–797. [Google Scholar]
- Patterson J.E., Andriole V.T. Bacterial urinary tract infections in diabetes, Infectious Disease Clinics of North America 9, 1995, 25–51. [PubMed] [Google Scholar]
- Rai U.S., Kumar U., Kumar H., Kumar O., Shahi Sk. Covert bacteriuria in nephrotic syndrome, Indian Journal of Pathologists and Microbiologists 45, 2002, 49–51. [PubMed] [Google Scholar]
- Rayfield E.J., Ault M.J., Keusch G.T., Brothers M.J., Nechemias C., Smith H. Infection and diabetes: the case for glucose control, American Journal of Medicine 72, 1982, 439–450. [DOI] [PubMed] [Google Scholar]
- Ronald A., Ludwig E. Urinary tract infections in adults with diabetes, International Journal of Antimicrobial Agents 17, 2001, 287–292. [DOI] [PubMed] [Google Scholar]
- Saitoh H., Nakamura K., Hida M., Sato T. Urinary tract infections in oliguric patients with chronic renal failure, Journal of Urology 133, 1985, 990–993. [DOI] [PubMed] [Google Scholar]
- Senior D.F. Management of difficult urinary tract infections. Bonagura J.D. Kirk's CVT XIII SA Practice, 2000, WB Saunders: Philadelphia, 883–886. [Google Scholar]
- Stamm W.E., Hooton T.M. Management of urinary tract infections in adults, New England Journal of Medicine 329, 1993, 1328–1334. [DOI] [PubMed] [Google Scholar]
- Stapleton A. Urinary tract infections in patients with diabetes, American Journal of Medicine 113 (Suppl 1A), 2002, 80S–84S. [DOI] [PubMed] [Google Scholar]
- Vinayagamoorthi R., Koner B.C., Kavitha S., Nandakumar D.N., Priya P. Padma, Goswami K. Potentiation of humoral immune response and activation of NF-B pathway in lymphocytes in experimentally induced hyperthyroid rats, Cellular Immunology 238, 2005, 56–60. [DOI] [PubMed] [Google Scholar]
- Wilson M.L., Gaido L. Laboratory diagnosis of urinary tract infections in adult patients, Clinical Infectious Diseases 38, 2004, 1150–1158. [DOI] [PubMed] [Google Scholar]