Abstract
Disseminated Mycobacterium avium-intracellulare complex (MAC) infection was diagnosed in 10 young cats (1–5 years of age) from Australia or North America between 1995 and 2004. A further two cats with disseminated mycobacteriosis (precise agent not identified) were recognised during this period. Of the 12, 10 were Abyssinian cats, one was a Somali cat and one was a domestic shorthair cat. None of the cats tested positive for either FeLV antigen or FIV antibody. The clinical course of these infections was indolent, with cats typically presenting for weight loss, initially in the face of polyphagia, with a chronicity of up to several months. Additional clinical features included lower respiratory tract signs and peripheral lymphadenomegaly. A marked diffuse interstitial pattern was evident in thoracic radiographs, even in cats without overt respiratory involvement. Hair clipped to perform diagnostic procedures tended to regrow slowly, if at all. Diagnosis was generally made by obtaining representative tissue specimens from mesenteric lymph nodes, liver or kidney at laparotomy, or from a popliteal lymph node. The primary antecedent event was most likely colonisation of either the alimentary or respiratory tract, followed by local invasion and eventual lymphatic and haematogenous dissemination. Nine cases were treated using combination therapy with agents effective for MAC infection in human patients. Two cats are still undergoing initial therapy and have responded. Of the remaining seven, all responded during long courses (5–14 months) of clarithromycin combined with either clofazimine or rifampicin, and a fluoroquinolone or doxycycline. Of these, three cats remain well (with durations between 2 months and 2 years following therapy); two developed recurrent disease (at 3 months and 2 years, respectively, following therapy) and have restarted therapy. The remaining two cats improved 1 year and 5 months, respectively, after diagnosis but ultimately succumbed. The two cats in which therapy was restarted have improved dramatically. Certain lines of Abyssinian and Somali cats likely suffer from a familial immunodeficiency that predisposes them to infection with slow-growing mycobacteria such as MAC.
Mycobacteria are aerobic, Gram-positive, non-motile bacilli with a high mycolic acid content in their cell wall that enables intracellular survival within mononuclear phagocytes. Being intracellular survivors, Mycobacteria species evoke a granulomatous to pyogranulomatous host response. Mycobacteria can be grouped conceptually into three categories: (1) obligate parasites that behave as primary pathogens and require a mammalian host to perpetuate their life cycle, including the species that comprise the tubercle bacilli (Mycobacterium tuberculosis, Mycobacterium bovis and Mycobacterium microti) and Mycobacterium lepraemurium, causal organism of a disseminated systemic disease in rats and feline leprosy (Hughes et al 1997, Greene and Gunn-Moore 1998, Malik et al 2000, 2001, 2002); (2) saprophytes that can behave as facultative pathogens, causing localised or systemic disease depending on the degree of host compromise; these can be divided further into slow growers, such as the Mycobacterium avium-intracellulare complex (MAC), Mycobacterium genavense and Mycobacterium xenopi, or rapid growers, such as Mycobacterium fortuitum, Mycobacterium chelonae, Mycobacterium smegmatis, Mycobacterium phlei and Mycobacterium thermoresistible; and (3) Mycobacteria species so difficult to culture that their environmental niche has not been determined with certainty, including Mycobacterium leprae, causal agent of leprosy in people and armadillos, the provisionally named Mycobacterium visibilis (Matthews and Liggitt 1983, Appleyard and Clark 2002), an as yet unnamed mycobacterial species recently shown to cause feline leprosy in older cats (Malik et al 2002, Hughes et al 2004) and the organism that causes the leproid granuloma syndrome in dogs (Hughes et al 2000).
The term MAC was coined after debate as to whether Mycobacterium intracellulare represented a distinct species or was merely a variant of M avium (Meissner et al 1974, Wayne et al 1981, Grange et al 1990). Traditionally, the MAC has been divided into serotypes using agglutination reactions, immunodiffusion, skin testing with sensitins, biochemical reactions on culture, or a combination of these (Grange et al 1990). Molecular techniques have more recently been used to clarify the MAC group (Thoresen and Saxegaard 1993, Ramasoota et al 2001).
M avium and related species are ubiquitous, saprophytic organisms commonly found in surface waters such as salt or fresh-water marshes, ponds, lakes, or soil. Animals, including people, are commonly exposed to these organisms (Grange et al 1990). M avium may give rise to disease if introduced in sufficient numbers through a breach in the skin or via alveolar deposition. In immune competent hosts, such events would cause localised infections, although generalised disease may arise in patients with compromised cell-mediated immunity (Horsburgh 1999).
In people, the most common immunodeficiency associated with disseminated MAC infection is the acquired immunodeficiency syndrome (AIDS). Other predisposing causes include autoimmune disease, neoplasia or immunosuppressive drug therapy (Grange et al 1990). In companion animals, most cases have been reported in animals less than 5 years old, with a marked overrepresentation of certain breeds, namely Siamese cats, Bassett Hounds and Miniature Schnauzers suggesting the possibility of an underlying familial immune defect (Hix et al 1961, Drolet 1986, Jordan et al 1994, Horn et al 2000). Such inherited familial immune defects have been well characterised in people and generally relate to defective interferon-gamma (IFN-γ) mediated immunity (Altare et al 1998, Remus et al 2001).
Of the 12 previously reported cases of disseminated MAC infection in cats (Hix et al 1961, Buergelt et al 1982, Drolet 1986, Morfitt et al 1989, Jordan et al 1994, Perkins et al 1995, van Dongen et al 1996, Latimer et al 1997, Barry et al 2002, Griffin et al 2003), none were treated successfully. In contrast, cats with disseminated disease attributable to other Mycobacteria species have responded favourably to treatment regimens incorporating clarithromycin (Gunn-Moore et al 1996, Malik et al 2002, Dietrich et al 2003).
The present series documents 12 cats with disseminated MAC infections, including nine cases that responded partially or completely to treatment. The marked preponderance of Abyssinian cats in this cohort suggests the likelihood of a specific familial immune defect, predisposing lines of this breed to disease caused by the M avium-intracellulare complex.
Results
All cases of disseminated MAC infection in cats that the authors became aware of during the study period were included. Two cases of disseminated mycobacteriosis in which the causative agent was not identified but the histology was similar to the other 10 cases were also included. No further similar cases were recognised.
Table 1 summarises the signalment, clinical findings, therapy and outcome for this cohort of patients. Table 2 summarises the pathological findings. All affected cats were 5 years of age or less at diagnosis and there was a marked preponderance of the Abyssinian breed (10/12) compared to a prevalence of approximately 1% in the Paddington Cat Hospital cat population (P<0.000001; two-tailed Fisher's exact test). Cases 8, 10 and 12 were siblings from different litters but all were the result of matings between case 9 and an unrelated unaffected queen. Cases 2 (Abyssinian cat) and 5 (the Somali cat) were from the same cattery as cases 8, 9, 10 and 12, but not closely related to case 9. All affected cats from this cattery had separate domiciles after being homed at approximately 12 weeks of age.
Table 1.
Signalment, pertinent findings, treatment and outcome of present series
| Case/country of origin | Year of diagnosis | Age at diagnosis (years) | Breed | Sex | Presenting complaint | Physical findings | Tissues involved | Lymph nodes affected | Mycobacterial therapy | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1, Australia | 1995 | 3 | Aby | Mn | Routine examination | Enlarged popliteal lymph node, subsequent weight loss | Intestines, lungs, liver, kidney, mesentery | Popliteal, mesenteric, medial iliac, hilar | Ciprofloxacin (125 mg bid) discontinued after 1 month | Euthanasia |
| 2, Australia | 1999 | 2 | Aby | Mn | Weight loss | Poor body condition, hepatomegaly, irregular kidneys, harsh inspiratory sounds | Lungs, liver, spleen, kidney | Mesenteric | Clarithromycin (62.5 mg bid), clofazimine (25 mg sid) | Resolved during 1 year therapy. Lost to follow-up 22 months later |
| 3, Australia | 1999 | 2 | DSH | Fs | Enlarged, reddenned vulva | Swollen, erythematous vulva, lymphadenomegaly (submandibular, popliteal, mesenteric) | lungs, vulva | Popliteal, submandibular, mesenteric | Clarithromycin (62.5 mg bid), clofazimine (50 mg eod) | Resolved during 1 year therapy. Recurrence 4 years later |
| 4, USA | 2000 | 3 | Aby | Fs | Weight loss, polyphagia | Poor body condition, harsh respiratory sounds, pyrexia | Ileum, liver, kidney, bone marrow, brain | Mesenteric, hilar | Not attempted | Euthanasia (diagnosis was post mortem) |
| 5, Australia | 2001 | 3 | Somali | Fs | Enlarged popliteal lymph node | Poor body condition, enlarged popliteal lymph node, subsequent weight loss | Lungs, liver, spleen | Popliteal, mandibular, mesenteric, others (not specified) | Not attempted | Euthanasia (diagnosis was post mortem) |
| 6, USA | 2002 | 4 | Aby | Fs | Weight loss | Poor body condition, dehydrated, pyrexia, harsh expiratory sounds | Lungs, liver, spleen, bone marrow | Mesenteric | Clarithromycin (30 mg bid) [adverse reactions to others tried] | Improved during therapy. Died 1 year later |
| 7, USA | 2002 | 5 | Aby | Mn | Weight loss, anorexia, tachypnoea | Poor body condition, abdominal mass, tachypnoea | Ileum, caecum, omentum, lungs | Mesenteric | Clarithromycin (15 mg bid) discontinued at 10 weeks, clofazimine (50 mg sid), doxycycline (50 mg bid) | Resolved during 5.5 months therapy. Therapy discontinued due to azotaemia |
| 8, Australia | 2003 | 1 | Aby | Mn | Weight loss, hyporexia | Poor body condition, enlarged popliteal lymph node, harsh respiratory sounds | Lungs | Popliteal | Clarithromycin (62.5 mg bid) adverse reaction to rifampicin (75 mg sid) after 4 months, changed to: clofazimine (25 mg sid) | Resolved during 14 months therapy |
| 9, Australia | 2004 | 4 | Aby | Mn | Weight loss, lethargy | Poor body condition, enlarged popliteal lymph node, bilateral renomegaly | Lungs, liver, spleen, kidney | Popliteal, mesenteric | Clarithromycin (62.5 mg bid), rifampicin (75 mg sid) | Clinically normal during 12 months of therapy. Recurrence 3 months later and therapy restarted |
| 10, Australia | 2004 | 2 | Aby | Mn | Ill thrift, anorexia, coughing, head tilt, ataxia | Poor body condition, harsh expiratory sounds, pyrexia, nystagmus | Liver, omentum, brain | Mesenteric, ileocaecal | Rifampicin (75 mg sid) possible adverse reaction to azithromycin (40 mg sid) after 4 months, changed to: clarithromycin (62.5 mg twice daily) | Initial improvement during therapy. Euthanased at 5 months due to deterioration |
| 11, USA * | 2004 | 4 | Aby | Mn | Weight loss, coughing, sporadic vomiting, blood in faeces | Poor body condition | Liver, spleen, pancreas, peritoneum | Mesenteric | Clarithromycin (62.5 mg bid), rifampicin (75 mg sid) | Improved. Still undergoing therapy (9 months of treatment at time of writing) |
| 12, Australia | 2004 | 2 | Aby | Mn | Weight loss, lethargy | Enlarged popliteal, mandibular lymph nodes, harsh respiratory sounds | Lungs | Popliteal, mandibular | Clarithromycin (62.5 mg bid), rifampicin (75 mg sid), doxycycline (50 mg sid) | Improved. Still undergoing therapy (7 months of treatment at time of writing) |
Aby=Abyssinian, DSH=domestic shorthair, Mn=male neuter, Fs=female spayed, bid=every 12 h, sid=every 24 h.
Moved to and was diagnosed in Australia.
Table 2.
Summary of abnormal laboratory findings in 11 cats with disseminated M avium
| Case | PCV (l/l) | Other haematology * | Blood biochemistry urinalysis * | FIV antibody | FeLV antigen | Cytology/histology | Organism identified |
|---|---|---|---|---|---|---|---|
| 1 | Normal | Unremarkable | Unremarkable | −ive | −ive | Popliteal lymph node cytology: large epithelioid macrophages containing numerous AFB. Lymph node histology: pyogranulomatous inflammation almost completely replacing normal lymph node architecture. Numerous beaded slightly curved AFB (1–4 μm in length) | M avium (culture) |
| 2 | 0.29 | Unremarkable | AST=83 U/l (0–65) | −ive | −ive | Liver, spleen and kidney histology: pyogranulomatous inflammation, rare AFB 2 μm in length, within macrophages | M avium (PCR and culture) |
| ALT=127 U/l (25–90) | |||||||
| 3 | 0.36 | WBC=25.3×109/l (6.0–16.0) | Unremarkable | −ive | −ive | Popliteal and mesenteric lymph node cytology: small lymphocytes with a marked increase in the number of macrophages, scattered neutrophils. No AFB seen (though cultured) | M avium (culture) |
| Neut (seg)=16.2×109/l (3.8–10.1) | |||||||
| Neut (band)=1.5×109/l (0–0.1) | |||||||
| Lymph=7.1×109/l (1.6–7.0) | |||||||
| 4 | 0.27 | WBC=14.2×109/l | Albumin=21 g/l (25–37) | −ive | −ive | Liver, kidney, bone marrow, brain and ileum histology: granulomatous inflammation with AFB | Not cultured |
| Mild neutrophilia | |||||||
| 5 | Not tested | Not tested | Not tested | −ive | −ive | Multifocal to coalescing areas of granulomatous inflammation within pleura, liver, spleen and multiple lymph nodes. Many of the foci have such advanced inflammation that the primary tissue of origin is obliterated. Rare AFB (3 μm in length) present, with beaded appearance | M avium (PCR) |
| 6 | 0.16 | WBC=52.8×109/l (4.2–15.6) | AST=91 U/l (5–55) | −ive | −ive | Spleen cytology: numerous AFB, some within neutrophils and macrophages. Abdominal fluid: modified transudate with equal numbers of neutrophils and macrophages, frequently containing abundant AFB | MAC (culture) |
| Neut (seg)=37.0×109/l (2.5–12.5) | ALT=338 U/l (12–130) | ||||||
| Neut (band)=7.9×109/l (0–0.3) | TP=96 g/l (59–85) | ||||||
| Mono=2.6×109/l (0–0.85) | Globulin=63 g/l (34–52) | ||||||
| Ca=2.8 mmol/l (1.9–2.7) | |||||||
| 7 | WBC=15.8×109/l (2.5–12.5) | Globulin=51 g/l (30–34) | −ive | −ive | Bronchial wash cytology and omentum and mesenteric lymph node histology showed pyogranulomatous inflammation, no AFB seen | M avium (culture) | |
| Neut (band)=1.9×109/l (0–0.3) | Ca=4.00 mmol/l (2.25–2.70) | ||||||
| iCa=1.59 mmol/l (1.22–1.30) | |||||||
| 8 | 0.37 | AST=107 U/l (26–43) | −ive | −ive | Popliteal lymph node histology: granulomatous inflammation with a predominance of activated macrophages with some neutrophils and occasional, beaded AFB 1–2.5 μm in length, within macrophages. | M avium (culture) | |
| WBC=23.4×109/l (5.5–19.5) | ALT=120 U/l (6–83) | ||||||
| Neut (seg)=14.0×109/l (2.5–12.5) | TP=80 g/l (54–78) | ||||||
| Mono=1.5×109/l (0–0.85) | Globulin=46 g/l (25–50) | ||||||
| Ca=2.65 mmol/l (1.5–2.60) | |||||||
| 9 | 0.43 | WBC=20.9×109/l (5.5–19.5) | Urea=5.32 mmol/l (5.71–12.85) | −ive | −ive | Liver, mesenteric lymph node histology: severe multifocal granulomatous inflammation with scattered AFB 1.5–2 μm in length (one organism measured at 4.5 μm) | Unsuccessful |
| UPC=2.38 | |||||||
| 10 | 0.25 | Neut (band)=1.01×109/l (0–0.3) | CK=252 U/l (50–200) | −ive | −ive | Liver and mesenteric lymph node histology: multifocal to coalescing areas of granulomatous inflammation with low numbers of AFB within the macrophages | M avium (PCR and culture) |
| Lymph=7.49×109/l (1.5–7.0) | AST=150 U/l (26–43) | ||||||
| ALT=94 U/l (6–83) | |||||||
| Ca=2.98 mmol/l (1.5–2.60) | |||||||
| 11 | 0.32 | WBC=23.0×109/l (5.5–19.5) | CK=406 U/l (50–200) | −ive | −ive | Extensive areas of chronic active granulomatous inflammation with distortion of normal organ architecture of pancreas, liver and lymph node. Rare AFB 2 μm in length, within mesenteric mass and lymph node | M avium (PCR) |
| Neut (seg)=14.7×109/l (2.5–12.5) | AST=117 U/l (26–43) | ||||||
| Lymph=7.36×109/l (1.5–7.0) | ALT=110 U/l (6–83) | ||||||
| Albumin=24 g/l (25–37) | |||||||
| Urea=6.6 mmol/l (7.0–10.7) | |||||||
| 12 | Not tested | Not tested | Not tested | Granulomatous to pyogranulomatous inflammation obliterating much of the normal nodal architecture. Extremely rare AFB, 1 μm in length | M avium (PCR) |
FIV=feline immunodeficiency virus, FeLV=feline leukaemia virus, −ive=negative, AST=aspartate aminotransferase, ALT=alanine aminotransferase, AFB=acid-fast bacilli, ZN=Ziehl-Neelsen stained sections, WBC=white blood cells, neut (seg)=segmented neutrophils, neut (band)=band form neutrophils, lymph=lymphocytes, Mono=monocytes, TP=total protein, Ca=total serum calcium, iCa=ionized serum calcium, CK=creatine kinase, UPC=urine protein to creatinine ratio, PCR=polymerase chain reaction.
Reference ranges given in parentheses, only abnormalities noted.
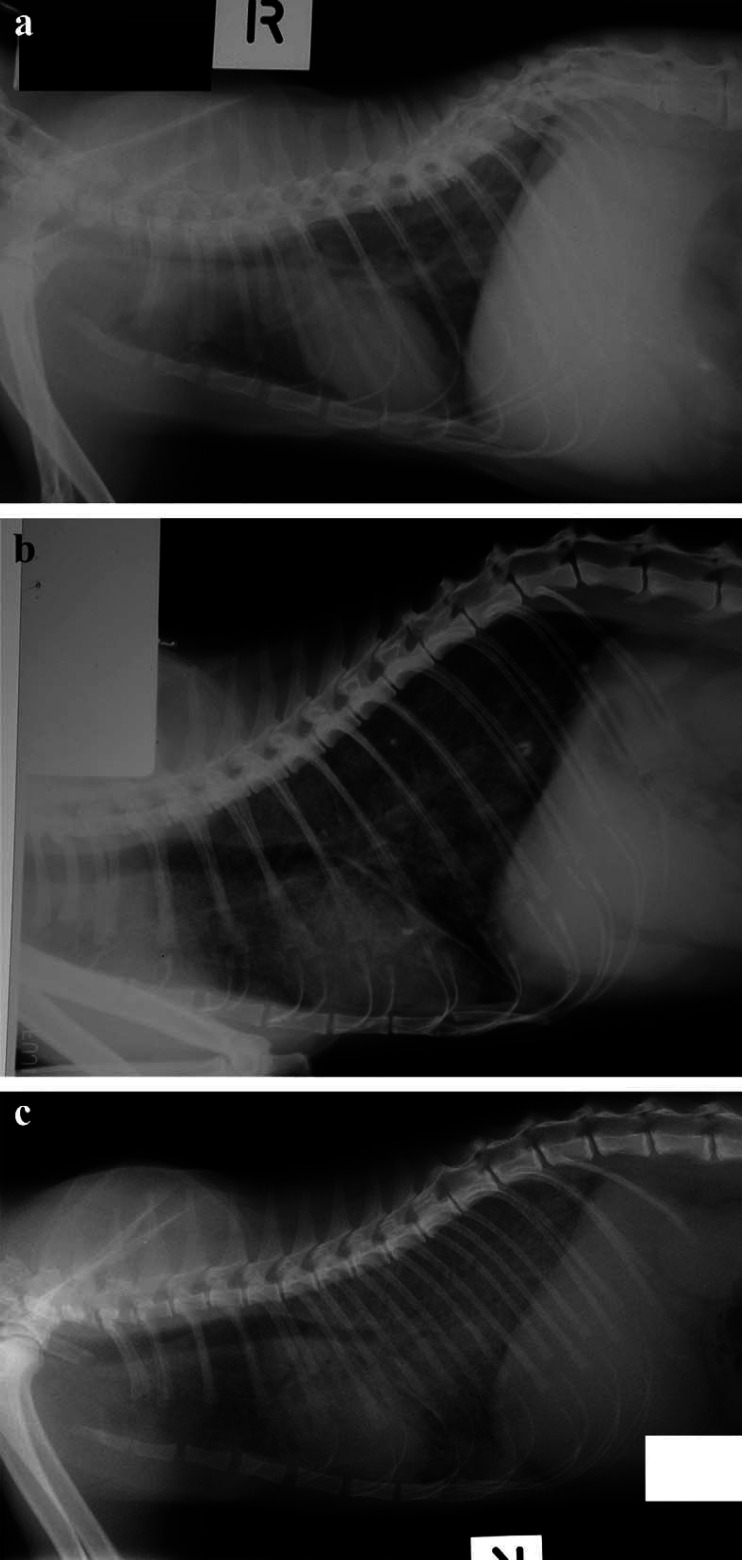
The most consistent physical findings were weight loss and ill thrift. Disease progression was slow, and in many cases, initial clinical findings were subtle or misleading. In some patients, signs were present for weeks or months before diagnosis. Thoracic radiographs were taken in 11 cases and demonstrated a distinctive severe diffuse interstitial pattern (Fig 1a–c) in cats with (7/7) and without pulmonary signs (2/4). Of the three cats tested, unguided broncho-alveolar lavage demonstrated pyogranulomatous inflammation in only one. Culture of the samples obtained was performed in two of these three cats and was unsuccessful in both. Other common clinical findings included enlarged popliteal lymph nodes (6/12) and lower respiratory tract signs such as coughing or dyspnoea (7/12). Hair clipped for diagnostic interventions often did not regrow until after therapy was initiated (Fig 2). Needle aspirates of peripheral lymph nodes did not always demonstrate granulomatous inflammation and organisms. Histological samples were required to secure a diagnosis in most cases.
Fig 1.
Lateral thoracic radiographs of cases 2, 3 and 8 (a, b and c, respectively), demonstrating a severe diffuse interstitial pattern which was remarkably similar in each patient. This pattern was characteristic of lung involvement in Abyssinian cats with disseminated M avium infections and presumably reflected haematogenous spread from a primary focus to the pulmonary parenchyma.
Fig 2.
Case 9 photographed 5 weeks after an exploratory laparotomy but before combination antimicrobial therapy. Note the poor regrowth of hair that had been clipped for surgery.
Clinicopathological findings were variable for the 12 cats: half showed a mild neutrophilia, one showed a dramatic neutrophilia with a left shift and results were unremarkable for the remainder. Only one cat (case 6) was anaemic and bone marrow infection was demonstrated in this case.
Serum biochemistry was largely unremarkable but sometimes reflected hepatic involvement with mild or moderate changes in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activity. Hypercalcaemia was noted in four cats. Three cats had poorly concentrated urine (USG<1.030) without azotaemia, while one cat with bilateral renomegaly (case 9) had marked proteinuria (urine protein:creatinine=2.38). All cats tested negative for FIV antibody and FeLV antigen. Many of the cats were investigated for gastrointestinal dysfunction but histology of the gastrointestinal tract demonstrated the aetiological agent in only three of nine cases subjected to biopsy. Interestingly, two cats showed evidence of inflammatory bowel disease, typically lymphocytic/plasmacytic enteritis.
One of the authors (MBK) reviewed the histology for the nine available cases. Typically, there were very low numbers of acid-fast bacilli (AFB) in lesions (ie, paucibacillary disease). In seven cases, rare or occasional AFB were evident and in two cases, no AFB could be detected despite a diligent examination of the sections from tissues that yielded a positive culture. In only two cases were numerous AFB evident microscopically (ie, multibacillary disease). The inflammatory response was invariably granulomatous to pyogranulomatous with significant infiltrates of lymphocytes, plasma cells and neutrophils. AFB in the sections varied in size from 1 to 4.5 μm in length with the majority of organisms 1–3 μm.
Antimycobacterial therapy was attempted in nine cats. All cases showed clinical improvement. The criteria for improvement were weight gain, improved laboratory results (in most cases to within reference range), demeanour and appetite. Four cats had completed therapy at the time of writing (cases 2, 3, 7, 8). Case 2 was apparently cured (body mass increased from 2.7 to 3.7 kg; no residual clinical signs) but was lost to follow-up 22 months after completing therapy. Case 3 developed recurrent disease 2 years after medication was discontinued; therapy was re-instituted and the cat again responded (body mass increased from 3.3 to 4.0 kg) after 6 months of treatment. Cases 7 and 8 have remained well 2 years (Sieber-Ruckstuhl et al, in preparation) and 2 months, respectively, after completing therapy. Case 6 showed clinical improvement (body weight improved from 3.1 to 3.7 kg) but died while undergoing additional diagnostics. Case 9 improved dramatically and completed 12 months of therapy but had a relapse 3 months later. Case 10 showed clinical improvement (weight gain from 2.4 to 5.1 kg) for 4 months but was euthanased 1 month later; it was unclear if the deterioration was related to mycobacterial disease and a necropsy was not permitted. Two cats (cases 11 and 12) are still undergoing therapy at the time of writing; at 9 and 7 months after starting therapy, both have shown clinical improvement.
Clarithromycin was the mainstay of therapy in all patients treated, generally at a dose of 62.5 mg per cat twice daily, but at a notably lower dose in case 6 that responded incompletely to therapy. The most commonly used additional anti-mycobacterial agents were clofazimine (25 mg once daily or 50 mg every other day) or rifampicin (75 mg once daily). Doxycycline (50 mg twice daily) was used as an additional agent in cases 6 and 12. A variety of adverse reactions (including pitting oedema, pyrexia, neurological signs and vomiting) were noted in number of cases, with clinical signs resolving after discontinuing the suspected agents (rifampicin, clofazimine or clarithromycin).
Three representative case reports are presented below to emphasise salient clinical features and diagnostic evaluation of these cases.
Case reports
Case 1
A 3-year-old castrated Abyssinian cat (5.1 kg) was presented for routine annual examination in 1995. Physical findings included an infected tooth root and enlargement of the left popliteal lymph node without evidence of any disease process affecting the distal left pelvic limb. Cytological examination of a Diff-Quik-stained aspirate from the lymph node revealed lymphoid hyperplasia (60% small lymphocytes, 40% large lymphocytes), increased numbers of plasma cells, occasional neutrophils, eosinophils and mast cells. The affected tooth was removed and a 6-day course of amoxicillin/clavulanate dispensed.
Two months later, the cat re-presented with further left popliteal lymphadenomegaly (2 cm in length). The cat was clinically well and had not lost weight. Physical examination was otherwise unremarkable. Diff-Quik-stained smears of lymph node aspirates, at this point, demonstrated a reactive lymph node with numerous large epithelioid macrophages containing numerous negatively-stained bacilli. Overstaining, using a modified acid-fast procedure (decolourising with 10% H2SO4 for 5 min), revealed large numbers of densely packed, beaded AFB 1–4 μm in length, within macrophages.
Haematology, serum biochemistry and thoracic radiographs were unremarkable. The cat tested FIV and FeLV negative. The affected lymph node was excised under general anaesthesia and the tissue divided into two portions for histological examination and microbiological studies, respectively. Empiric therapy was started using ciprofloxacin (125 mg orally twice daily). Mycobacteria were first detected on blood agar after 11 days of incubation. The isolate was identified as M avium at a reference laboratory (Centre for Infectious Diseases and Microbiology, Westmead Hospital, Australia).
The cat presented for aural pruritus 1 month after commencing ciprofloxacin. Both pinnae and the ventral abdomen were erythematous and the cat was pyrexic (39.7°C). An adverse reaction to ciprofloxacin was suspected and it was discontinued. Erythema and pyrexia resolved but unfortunately, the owner was reticent to treat the cat further with multi-agent therapy more appropriate for M avium.
Three months after discontinuing ciprofloxacin (ie, 6 months after the enlarged lymph node was first detected), the cat was presented for poor appetite and weight loss of 1.0 kg. Additional physical findings included pyrexia (39.6°C) and an irregular, firm, non-painful mid-abdominal mass. Following sedation, aspiration of this mass yielded a specimen containing enormous numbers of AFB within and around epithelioid macrophages. The owner declined therapy and elected euthanasia.
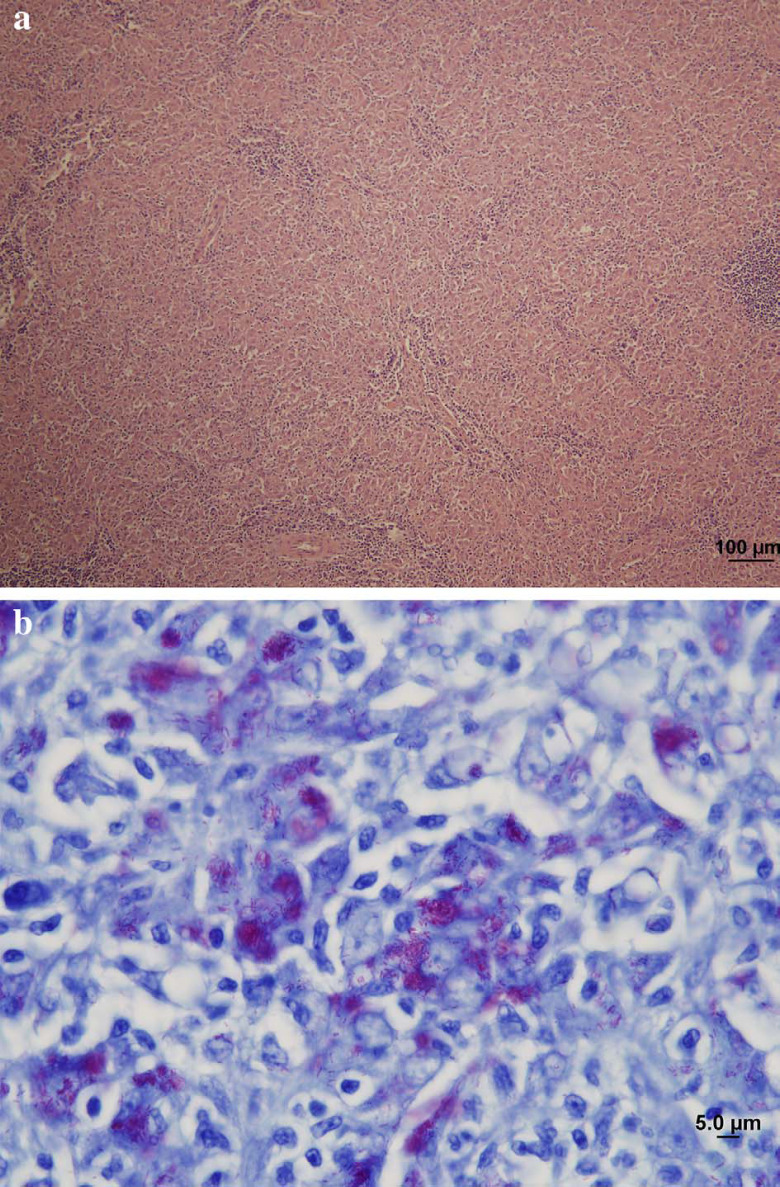
Necropsy examination revealed multiple white nodules in the intestinal wall, liver, kidney, spleen, lungs and the mesenteric, medial iliac and hilar lymph nodes. Histology of an affected lymph node (Fig 3a and b) demonstrated sheets of epithelioid macrophages with abundant eosinophilic cytoplasm containing variably sized small vacuoles. Many of these macrophages contained large numbers of AFB consistent with the appearance of mycobacteria. Neutrophils, lymphocytes and plasma cells were interspersed amongst the epithelioid macrophages with occasional small foci of neutrophilic aggregation. Efferent lymphatics also contained sheets of epithelioid macrophages.
Fig 3.
Low power photomicrograph (a) of the enlarged popliteal lymph node of case 1. The normal lymph node architecture has been completely effaced by sheets of epithelioid macrophages, with only scattered lymphocytes remaining. Haematoxylin and eosin (H & E) stain; scale bar=100 μm. A high power photomicrograph (b) of the same lymph node, stained using the Ziehl-Neelsen method, showing the less common scenario of enormous numbers of intracellular acid-fast bacilli (staining pink with the carbol fuchsin) in the majority of macrophages. Such multibacillary lesions were less common in our series than paucibacillary lesion. Note that the acid-fast bacilli are short, averaging 3 μm in length. Scale bar=5 μm.
Case 2
A 2-year-old castrated Abyssinian cat was presented in 1999 with a 2-month history of weight loss, polyphagia and sporadic vomiting. The cat's diet consisted of a mixture of balanced commercial canned and dry foods and its faeces were normal. The cat had been examined by another veterinarian and found to be pyrexic. It had been treated with various anthelminthics, ketoprofen, amoxicillin clavulanate and corticosteroids.
The cat was thin (2.7 kg) with an unusual barrel-shaped chest and a mild fever (39.3°C). Physical findings included hepatomegaly, irregular kidneys and occasional harsh inspiratory sounds on thoracic auscultation. Hair that had been clipped for investigations 6 weeks earlier had not regrown. History and physical findings suggested a single disease process affecting many organs or multiple disorders.
Haematology was unremarkable. Serum biochemical testing revealed mild elevations of aspartate aminotransferase (AST) (83 U/l; reference range [RR]: 0–65 U/l) and alanine aminotransferase (ALT) (127 U/l; RR: 25–90 U/l) activities. Urinalysis showed a specific gravity of 1.020 with trace protein. Serum thyroxine concentration was normal. Immunomigration tests for FIV antibody and FeLV antigen were negative.
Thoracic radiographs showed a diffuse bronchointerstitial pattern (Fig 1a). Abdominal radiographs demonstrated hepatosplenomegaly and presumed congenital lumbar vertebral malformations. A disseminated infectious or neoplastic condition, involving the lower respiratory tract, liver, spleen and kidneys, appeared likely. Broncho-alveolar lavage using a technique similar to that described by McCauley et al (1998) produced a sample with light wisps of mucus, containing numerous inflammatory cells (75% neutrophils, 15% broncho-alveolar macrophages, 10% lymphoid cells [mainly small lymphocytes]) and scattered ciliated columnar epithelial cells. Unfortunately, the specimen was not cultured.
Exploratory laparotomy was performed 2 days later. Hepatomegaly and splenomegaly were confirmed and enlargement of mesenteric lymph nodes was apparent. Biopsy specimens were obtained from the liver, spleen, kidney, jejunum and mesenteric lymph node and fixed in neutral buffered formalin for histologic examination. A fresh portion of liver was submitted for cytological and microbiological studies.
Modified acid-fast stained cytological preparations of liver revealed rare scattered AFB 2 μm in length. Histologically, there was pyogranulomatous to granulomatous inflammation of the liver, spleen and kidney. Occasional AFB were detected within the cytoplasm of some macrophages in Ziehl-Neelsen stained sections of liver, spleen and kidney (Table 2).
Following tituration, the fresh liver specimen was inoculated on to blood agar and Ogawa egg yolk-medium at 28°C and 37°C. A portion of the liver sample was also forwarded to the same reference laboratory as case 1. A mycobacterial species was first detected on blood agar after 11 days of incubation. This was later identified as M avium by high performance liquid chromatography analysis of mycolic acids (Butler and Guthertz 2001). PCR produced an amplicon with a 16S rRNA sequence compatible with M avium.
Amoxicillin clavulanate (52.5 mg) and enrofloxacin (15 mg) were administered subcutaneously once daily while awaiting laboratory results. When food was offered 2 days later, antibiotic therapy was continued orally (amoxicillin clavulanate 50 mg twice daily; enrofloxacin 25 mg once daily).
The cat's demeanour improved while receiving this therapy. Hair that had been clipped by the previous veterinarian started to regrow. The cat, however, did not gain weight. Specific anti-mycobacterial drug therapy consisting of clarithromycin (62.5 mg orally twice daily), rifampicin (50 mg orally once daily) and enrofloxacin (25 mg once daily) was commenced 7 days following surgery. Twelve days after starting this regimen, the cat became lethargic. Pitting oedema of the front paws was evident on physical examination; the cat had difficulty in walking and was pyrexic (39.5°C). An adverse reaction to rifampicin was suspected, so clofazimine (25 mg once daily) was substituted.
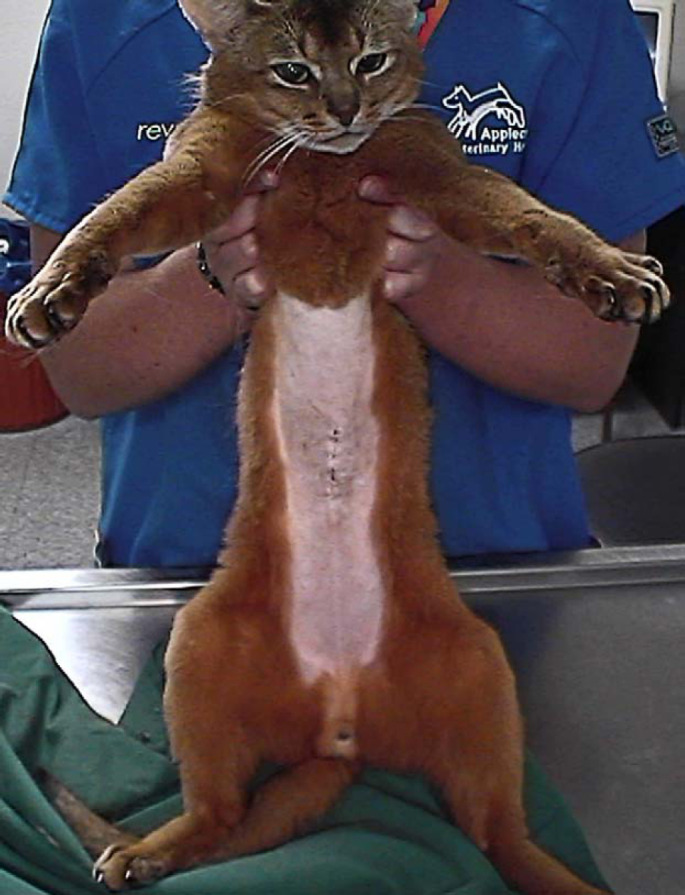
Treatment was discontinued after 3 months due to poor compliance. The cat represented approximately 6 months later with recurrence of the initial presenting complaints. It was again treated with clarithromycin (62.5 mg twice daily) and clofazimine (25 mg once daily). Clinical signs improved within a month and treatment was continued for a full year. The cat (3.7 kg) was clinically normal (Fig 4) 22 months after therapy was discontinued, at which time the cat and owner moved overseas and were lost to further follow-up.
Fig 4.
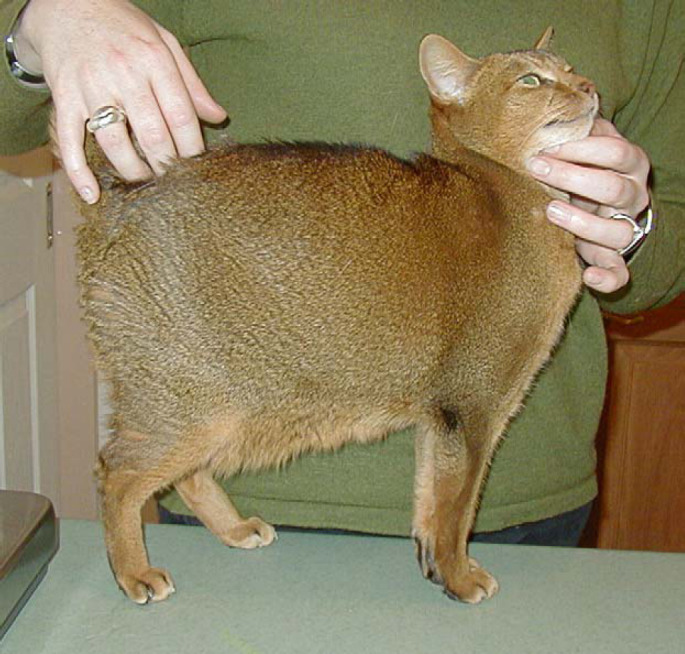
Case 2, 2 years after completing therapy. Note the excellent body condition and the normal hair coat.
Case 8
A 1-year-old male castrated Abyssinian was presented for weight loss and inappetence in 2003. The cat was thin (2.7 kg) and had an enlarged left popliteal lymph node.
Haematology revealed a mature neutrophilia, (Table 2). Serum biochemistry revealed mild elevations of AST (107 U/l; RR: 26–43 U/l) and ALT (120 U/l; RR: 6–83 U/l) activities and hypercalcaemia (total calcium=2.7 mmol/l; RR: 1.5–2.6 mmol/l).
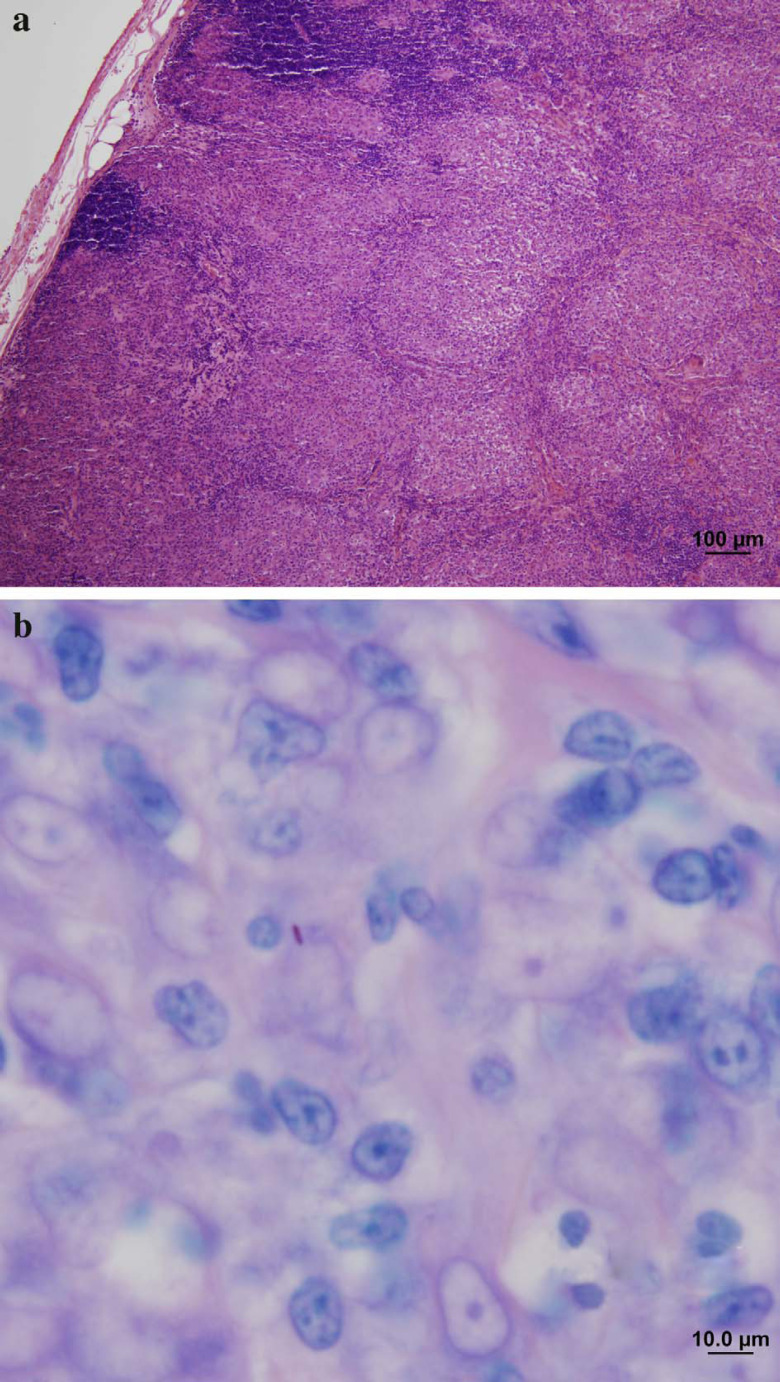
Thoracic radiography revealed a marked diffuse interstitial pattern (Fig 1c). Histology of a biopsy sample of the affected lymph node showed pyogranulomatous inflammation and some reactive fibroplasia. Occasional, 1–2.5 μm long, beaded AFB were seen intracellularly within macrophages in Ziehl-Neelsen stained sections (Fig 5a and b).
Fig 5.
Low power photomicrograph (a) of an enlarged lymph node from case 8. Lymph node architecture has been partially effaced by sheets of epithelioid macrophages. Only a remnant of the normal cortical histological architecture remains. H & E stain; scale bar=100 μm. Higher power Ziehl-Neelsen stained section (b) of the same lymph node showing the more common paucibacillary reaction pattern; only one acid-fast bacillus (stained red, just left of centre) can be seen; scale, bar=10 μm.
A week later, the cat was anaesthetised again so samples could be collected from the affected popliteal lymph node for culture at a mycobacteria reference laboratory (Western Australian Centre for Pathology and Medical Research, Queen Elizabeth II Medical Centre). M avium was cultured. While awaiting results, anti-mycobacterial therapy was started using clarithromycin (62.5 mg twice daily) and rifampicin (75 mg once daily).
The cat initially improved markedly with regrowth of shaved hair and weight gain. Thoracic radiographs were normal 3 months after starting therapy. The clinical course was subsequently punctuated by episodes of lethargy and apparent abdominal pain. Much of the regained weight was lost. Rifampicin was discontinued and clofazimine (25 mg once daily) was substituted. The cat's clinical improvement continued for a further 4 months until the cat became difficult to medicate and weight loss again recurred. The cat was hospitalised for a week to re-institute treatment and the cat improved again. Clofazimine became unavailable after a further 4 months (11 months after initiating therapy) and the cat was continued on clarithromycin alone for a further 3 months (totalling 14 months of therapy). The cat remains well and weighed 4.4 kg 2 months after discontinuation of medication.
Discussion
This is a substantial case series of 12 disseminated M avium infections in cats, particularly considering that over the last 44 years there have been only a total of 12 cases recorded in the literature (Hix et al 1961, Buergelt et al 1982, Drolet 1986, Morfitt et al 1989, Jordan et al 1994, Perkins et al 1995, van Dongen et al 1996, Latimer et al 1997, Barry et al 2002, Griffin et al 2003; see Table 3). It is striking that most of the present cases are Abyssinian cats, a breed not recognised as being predisposed to this condition. Furthermore, at least four cases had a traceable common familial relationship. We record, for the first time, the successful treatment of a substantial proportion of cats using combination therapy incorporating clarithromycin.
Table 3.
Signalment and clinical features of prior reports of cats with disseminated infection with slow-growing opportunistic mycobacteria
| Reference | Age (years) | Sex * | Breed | Presenting complaint | Physical findings | Retrovirus status (by ELISA) | PCV (l/l) | Culture | |
|---|---|---|---|---|---|---|---|---|---|
| FeLV | FIV | ||||||||
| Hix et al (1961) | 4 | F | Siamese | Coughing, weight loss, anorexia, lethargy | Pyrexia, emaciation, deydration | NR | NR | 0.22 | M avium |
| Buergelt et al (1982) | 13 | F | Domestic | Subcutaneous nodules | Pyrexia, lymphadenomegaly, dyspnoea | −ive | NR | Normal | M avium serotype 1 |
| Drolet (1986) | 5 | M | Siamese | Decreased appetite, mandibular lymphadomegaly | Pyrexia | −ive | NR | 0.08 | M avium |
| Morfitt et al (1989) | 7/12 | M | DSH | Anorexia, respiratory signs | Pyrexia, lymphadenopathy | −ive | −ive | 0.17 | M avium serotype 1 |
| Jordan et al (1994) | 1.5 | F | Siamese | Anorexia, lethargy | Thin, recumbent, splenomegaly, other abdominal masses | −ive | −ive | 0.15 | MAC |
| Jordan et al (1994) | NR | F | Siamese | Anorexia, abdominal tenderness | Pyrexia, thin, mesenteric lymphadenomegaly | −ive | −ive | 0.16 | MAC |
| Jordan et al (1994) | 2 | F | Siamese | Anorexia, vomiting, weight loss | pyrexia, thin, tachypnoea, lymphadenomegaly | −ive | −ive | 0.18 | M avium |
| Perkins et al (1995) | 11 | M | DLH | Cachexia, icterus, diarrhoea | Not noted | NR | NR | 0.22 | M avium |
| van Dongen et al (1996) | 2 | M | DSH | Lymphadenopathy, lethargy, weight loss | Generalised lymphadenomegaly | −ive | −ive | 0.19 | M avium serotype 2 |
| Latimer et al (1997) | 4 | M | DSH | Weight loss, sporadic vomiting | Pyrexia, thin, dehydration, abdominal organomegaly, expiratory lung sounds | −ive † | −ive | 0.09 | MAC |
| Barry et al (2002) | 7 | F | DSH | Weight loss, difficulty walking | Pyrexia, muscle wastage, hind limb ataxia | −ive | −ive | 0.25 | M avium |
| Griffin et al (2003) | 11 | F | DSH | Weight loss, lethargy, hyporexia, vomiting, diarrhoea, haematochezia | Unkempt coat, pyrexia, systolic cardiac murmur | −ive | −ive | 0.21 | M avium |
| MacWilliams et al (1998) | 4 | F | DSH | Submandibular lymphadomegaly, inappetence, lethargy | Pyrexia, thin, abdomen distention (ascites), abdominal masses | −ive | −ive | Normal | M xenopi |
| Hughes et al (1999) | 8 | M | Siamese | Coughing, periocular alopecia, inappetence | Pyrexia, poor dry hair coat, thoracic crackles | −ive | +ve | 0.30 | M genavense |
| Dietrich et al (2003) | 8 | M | DSH | Nodules on limb, dyspnoea, coughing, right pupil dilated | Multiple intra and subcutaneous lesions, poor coat, harsh lung sounds generalised lymphadomegaly, poor reflexes in dilated right pupil | −ive | −ive | Normal | M simiae |
| Cats with documented systemic mycobacteriosis without identification of the causative agent | |||||||||
| Wolff (1966) | 1 | NR | DSH | Diarrhoea, anorexia | Dehydration | NR | NR | NR | |
| Grossman (1983) | 3 | M | Abyssinian | Coughing | Thin, moist lung sounds, hepatomegaly, | −ive | NR | NR | |
| Evans and Caylor (1995) | 1 | F | Himalayan | Swelling on both sides of neck | Submandibular lymphadomegaly, later weight loss | −ive | NR | NR | |
| Pinson and Tucker (1998) | 2 | F | DSH | Progressive weight loss, subcutaneous masses | Cachexia, generalised lymphadomegaly | NR | NR | NR | |
NR=not recorded, DSH=domestic shorthair, DLH=domestic longhair, MAC= M avium complex.
All cases desexed. F=female, M=male.
FeLV positive by BM IFA.
Of the 12 previously reported cases of disseminated MAC infection in cats, nine were single case reports (Hix et al 1961, Buergelt et al 1982, Drolet 1986, Morfitt et al 1989, Perkins et al 1995, van Dongen et al 1996, Latimer et al 1997, Barry et al 2002, Griffin et al 2003; see Table 3). MAC infection of the CNS was observed in an additional cat and although signs of systemic disease were not detected, CNS involvement was presumed to have resulted from haematogenous spread from an undetected primary focus (Blauvelt et al 2002). Disseminated mycobacteriosis has also been documented with other slow-growing Mycobacterium species: M xenopi (MacWilliams et al 1998), M genavense (Hughes et al 1999) and M simiae (Dietrich et al 2003). Systemic mycobacteriosis has been documented in a further four cats in which the causative agent was not identified to the species level (Wolff 1966, Grossman 1983, Evans and Caylor 1995, Pinson and Tucker 1998). Of the previously reported cases with disseminated mycobacteriosis caused by MAC, there was a preponderance of Siamese cats (5/12) (Hix et al 1961, Drolet 1986, Jordan et al 1994). Likewise, 15 of 80 cats with tuberculosis (M bovis) in a Swiss study were Siamese (Hix et al 1961) suggesting that this breed may be at increased risk for developing systemic mycobacteriosis. Additionally, of the four cats with unspecified systemic mycobacteriosis, one case was an Abyssinian cat. As in the present case series, most reported cats with disseminated mycobacteriosis due to slow-growing Mycobacterial species, were less than 5 years old (12/19 for which age was recorded).
Disseminated disease attributable to slow-growing, saprophytic Mycobacteria species generally reflects defective cell-mediated immunity. FeLV antigen was recognised in the bone marrow of one MAC-infected cat using immunofluorescence (Latimer et al 1997). FIV was identified in a cat with sequential opportunistic infections that eventually succumbed to a disseminated M genavense infection (Hughes et al 1999). Another cat acquired a disseminated M avium infection while receiving cyclosporine following renal transplantation (Griffin et al 2003). Deficits in cell-mediated immunity also occur secondarily to other immunosuppressive drugs (eg, corticosteroids), malignancy, malnutrition, old age, splenectomy and endocrinopathies (Thompson 1994). Most of these causes of immunodeficiency are unlikely in a cohort of young cats with disseminated MAC infections. Genetic susceptibility to intracellular pathogens, due to an inherited immune defect, represents a much more plausible possibility.
In some families of mice, there is a single autosomal gene, Bcg, which codes for resistance to M tuberculosis and other intracellular pathogens such as leishmania and salmonella (Sifford and Bates 1991). Predisposition to disseminated MAC infection in association with reduced cellular immune reactivity has been documented in a species of tree Kangaroo (Montali et al 1998). In people, Mendelian susceptibility to poorly virulent Mycobacteria species comprises a heterogenous syndrome, with affected children having one of a number of different mutations affecting IFN-γ mediated immunity. In cases with partial receptor deficiencies or lack of IFN-γ, exogenous administration of this cytokine is an important component of therapy in addition to antibiotics (Altare et al 1998, Remus et al 2001). It seems plausible that certain lines of Abyssinian and Siamese cats suffer from a similar immunological defect. Furthermore, an increased incidence of cryptococcosis and blastomycosis has been noted in both breeds (Davies and Troy 1996).
The pathogenesis of disseminated MAC infection has not been established in cats. The location and distribution of mycobacterial lesions likely reflects the route of primary exposure. Dissemination probably arises following (i) ingestion of contaminated soil or water or (ii) inhalation of contaminated airborne dust by immunodeficient individuals (Greene and Gunn-Moore 1998). In people, infection is acquired by ingestion (90% of cases) or inhalation (10%) with subsequent gastrointestinal or pulmonary colonisation. After colonisation, organisms penetrate the epithelium to reach the gastrointestinal lamina propria or pulmonary interstitium where phagocytosis by macrophages should normally occur. In susceptible individuals, the failure of intracellular killing permits local replication and subsequent haematogenous dissemination, via infected mononuclear phagocytes. The most frequent distant sites of infection in people are reticuloendothelial organs including liver, spleen and sometimes bone marrow (Horsburgh 1999). Cats appear to be affected similarly. The disparate clinical and pathological findings found in this series most likely reflect haematogenous spread. Unlike M lepraemurium, M microti, M genavense, M visibilis and the novel feline leprosy organism, skin involvement is uncommon, whereas lymph nodes are frequently involved. The large number of cats with lower respiratory involvement suggests the possibility of primary inhalation exposure in these individuals. On the other hand, the interstitial lung pattern and the minimal aid to diagnosis from BAL is more compatible with haematogenous dissemination rather than alveolar deposition.
The majority (11 of 12) of previously reported cases were anaemic. Only one cat (case 6) in this series had anaemia, possibly attributable to bone marrow involvement. Anaemia of chronic disease is common in people with disseminated MAC infection, organisms are rarely seen in bone marrow (Horsburgh 1999). Any serum biochemistry changes of either prior reports or the current series were inconsistent and mostly minimal. Hypercalcaemia, seen in far cats of this series, likely reflected granulomatous inflammation (Mealey et al 1999).
Imaging studies may assist in the diagnosis of these cases by demonstrating intra-abdominal lymph node enlargement, hepatosplenomegaly or lung involvement, as in the present case series. Multi-system disease with organomegaly and lymphadenomegaly is strongly suggestive of infiltrative disease. In cats, diagnostic possibilities include neoplasia (eg, lymphosarcoma and mastocytosis), chronic inflammation (eg, feline infectious peritonitis, mycobacteriosis or systemic mycoses), hypereosinophilic disease, or possibly a tissue deposit (eg, amyloidosis). Definitive diagnosis of disseminated mycobacteriosis requires visualisation of AFB associated with granulomatous or pyogranulomatous inflammation and positive culture or molecular identification. In most cases of the present series, diagnosis was achieved only after histological examination and microbiological assessment of formalin-fixed and fresh specimens, respectively, of affected tissues taken at laparotomy. However, with a higher index of suspicion, it is possible that cases could be diagnosed using needle aspirate cytology, culture and molecular methods such as PCR. Real-time PCR has recently been shown to be useful for this purpose in people (Bruijnesteijn van Coppenraet et al 2004).
In the current series, two cats (cases 6 and 11) showed evidence of inflammatory bowel disease (lymphocytic/plasmacytic enteritis) but without macrophage infiltration or any evidence of an aetiological agent. This finding is potentially misleading, as treatment of such cases with corticosteroids would be expected to further impair cell-mediated immunity, thereby permitting the mycobacterial infection to disseminate further. We suspect these cases represent infection elsewhere along the gastrointestinal tract or heavy colonisation of the intestinal lumen.
Treatment of mycobacterial disease poses several difficulties. To work, antimicrobials must reach therapeutic concentrations within phagocytes in various tissues, but with minimal toxicity to the host. Importantly, there is a propensity for Mycobacteria species in general, and MAC organisms in particular, to spontaneously and rapidly develop antibiotic-resistant mutants (Masur 1993). Multiple agents should, therefore, be used to reduce the chance of resistant clones developing. Using several agents concurrently, however, increases the likelihood of adverse drug reactions, as each agent has a potential toxicity profile. Furthermore, some of these profiles overlap (Masur 1993).
Although localised MAC infections in cats have been managed successfully (Kaufman et al 1995, Deykin et al 1996, Malik et al 1998), successful treatment of disseminated M avium infection in cats has not been reported. Other systemic mycobacterial infections have been successfully treated (Gunn-Moore et al 1996, Appleyard and Clark 2002, Dietrich et al 2003). Attempted but unsuccessful drug regimens in previously reported disseminated MAC cases utilised ciprofloxacin and rifampicin (Jordan et al 1994), enrofloxacin and rifampicin (van Dongen et al 1996), clofazimine, doxycycline and an unspecified interferon (Latimer et al 1997) and enrofloxacin and azithromycin (Barry et al 2002). In all cases except one, the patients died within 3 days of initiating therapy, a time frame so short that they cannot really be considered therapeutic failures. In the remaining case (van Dongen et al 1996), the patient improved, however, lymphadomegaly persisted and the cat was euthanased after 1 year. Necropsy revealed numerous granulomatous inflammatory lesions containing Mycobacteria species and substantial calcification in many internal organs (van Dongen, personal communication).
In people with disseminated MAC disease, clarithromycin is considered the preferred primary antibiotic (Chaisson et al 1994). In the present series of cases, all cats that improved (including those with complete resolution of signs) received clarithromycin, mostly at a dose of 62.5 mg/cat twice daily. The most commonly used additional anti-mycobacterial agents were clofazimine (25 mg once daily or 50 mg every second day) or rifampicin (50–75 mg once daily). Adverse reactions were noted in a number of cases with these events resolving after discontinuing the suspected agent.
Clarithromycin is a macrolide antibiotic that reaches high concentrations in the respiratory tract and concentrates intracellularly within macrophages and neutrophils, thereby facilitating killing of intracellular pathogens (Greene and Watson 1998). Clarithromycin appears to have selective bactericidal action against M avium, and this tendency is increased when it is used in combination with other agents (Rastogi and Labrousse 1991). Although pharmacokinetic data in cats is lacking, its use has been documented (Gunn-Moore et al 1996, Barrs et al 1999, Foster et al 1999, Malik et al 2001, 2002). No adverse effects have been recognised in those reports but one of the authors (SF) has observed reversible hepatotoxicity in one patient as noted in case 7 (Sieber-Ruckstuhl et al, in preparation).
Clofazimine is a dye that binds preferentially to mycobacterial DNA. It is highly lipophilic and thus taken up by adipose tissue and mononuclear phagocytes (Greene and Watson 1998). It has previously been used in cats (both alone and in combination) to treat ‘leprosy’ (Malik et al 1998, 2001, 2002, Barrs et al 1999), localised MAC infections (Kaufman et al 1995, Malik et al 1998) and mycobacterial panniculitis (Michaud 1994). Adverse reactions in cats include hepatotoxicity, inappetence, weight loss, skin discoloration and photosensitivity; all of which are reversible after discontinuation of therapy (Kaufman et al 1995, Malik et al 1998, 2002, Barrs et al 1999). Case 6 vomited on each occasion that clofazimine was administered.
Rifampicin inactivates bacterial DNA-dependent RNA polymerase. It has high lipid solubility and penetrates most tissues. Importantly, it is effective against organisms in extracellular cavitary spaces and caseous lesions (Greene and Watson 1998). Rifampicin has been used to treat various feline mycobacterial infections (Gunn-Moore et al 1996, Foster et al 1999, Malik et al 2002). Potential adverse reactions include hepatotoxicity, CNS disturbances, pinnal erythema, pruritis and dyspnoea (Greene and Watson 1998). In case 2, pitting oedema of the front paws (suggestive of vasculitis) was noted in association with pyrexia and possible CNS signs, all of which resolved after its discontinuation.
Although older fluoroquinolones such as ciprofloxacin and enrofloxacin are considered acceptable for treating localised infections due to rapidly-growing mycobacteria (Studdert and Hughes 1992, Malik et al 2001), numerous authors have concluded they are ineffective for the treatment of MAC infections in human patients (Alangaden and Lerner 1997, Tomioka et al 2002) and it is difficult to ascertain whether they make any contribution to therapy when used in combination with other agents. Thus traditional fluoroquinolones probably have little or no place as part of an antimicrobial regimen for feline MAC infections, despite their widespread availability and familiarity, although some newer fluoroquinolones such moxifloxacin may prove useful in the future (Caeiro and Iannini 2003).
Use of combination therapy is vital for successful treatment of MAC infections. As well as minimising the possibility of resistance developing (Masur 1993), combination therapy can produce synergistic effects. For example, Tomioka et al (2002) demonstrated that clarithromycin potentiated the activity of rifampicin against both extracellular and intracellular MAC.
In conclusion, despite the presence of widely disseminated disease at presentation, a successful outcome is possible using appropriate combination therapy, modified in the light of adverse drug events and the response to therapy. From the authors' evolving experience, we currently recommend clarithromycin to be used with either clofazimine or rifampin as first line therapy for disseminated MAC infection in cats. In the future, additional agents such as moxifloxacin or linezolid may also establish a place in the treatment of these cases.
Acknowledgements
The authors would like to acknowledge the assistance or Dr Sue Foster for the management of Case 2. We are very appreciative of Drs Ingrid Bey (Anderson Lakes Animal Hospital, Minnesota, USA), Micaela Elphick (Doyalson Animal Hospital, NSW, Australia), Marian McFetridge (Warton Road Small Animal Hospital, WA, Australia), Kathy Moylan (Alice Springs Veterinary Clinic, NT, Australia) and Suzy Moore (Melville Animal Hospital, WA, Australia) for providing cases for this study and making their clinical records accessible. We would also like to thank Dr John Jardine from Vetpath Laboratory Services (Western Australia) for providing histological samples from a number of the cases to make the histopathological review possible.
Addendum
Clofazimine (“Lamprene”®) has not been available through traditional pharmaceutical distribution since late 2004. This is because Novartis, the World Health Organization and the U.S. Department of Health and Human Services together determined that clofazimine would only be available for the treatment of Hansen's Disease (human leprosy). Thus for veterinary usage, clofazimine is currently available only via compounding pharmacists.
Since this article was submitted, case 9 has continued to improve. This was thought to be at least partially attributable to addition of moxifloxacin (50mg orally once daily) to the therapeutic regimen. This quinolone is used in some human patients with M avium infections, and may prove useful for treatment of a variety of mycobacterial disease in the cat, especially given the limited availability of clofazimine.
Prior reports of disseminated MAC infection in dogs have noted lung involvement but a recent case report (Naughton et al 2005) describes thoracic radiographic changes remarkably similar to the cats present report.
Finally, a further case of disseminated MAC infection in a cat has been reported since this paper was originally submitted (Knippel et al 2004).
References
- Alangaden G.J., Lerner S.A. The clinical use of fluoroquinolones for the treatment of mycobacterial diseases, Clinical Infectious Diseases 25, 1997, 1213–1221. [DOI] [PubMed] [Google Scholar]
- Altare F., Jouanguy E., Lamhamedi S., Döffinger R., Fischer A., Casanova J.-L. Mendelian susceptibility to mycobacterial infection in man, Current Opinion in Immunology 10, 1998, 413–417. [DOI] [PubMed] [Google Scholar]
- Appleyard G.D., Clark E.G. Histologic and genotypic characterization of a novel Mycobacterium species found in three cats, Journal of Clinical Microbiology 40, 2002, 2425–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrs V.R., Martin P., James G., Chen S., Love D.N., Malik R. Feline leprosy due to infection with a novel mycobacterial species, Australian Veterinary Practitioner 29, 1999, 159–164. [Google Scholar]
- Barry M., Taylor J., Woods J.P. Disseminated Mycobacterium avium infection in a cat, Canadian Veterinary Journal 4, 2002, 369–371. [PMC free article] [PubMed] [Google Scholar]
- Blauvelt M., Weiss D., McVey A., Bender J., Aird E. Space-occupying lesion within the calvarium of a cat, Veterinary Clinical Pathology 31, 2002, 19–21. [DOI] [PubMed] [Google Scholar]
- van Coppenraet E.S. Bruijnesteijn, Lindeboom J.A., Prins J.M., Peeters M.F., Claas E.C.J., Kuijper E.J. Real-time PCR assay using fine-needle aspirates and tissue biopsy specimens for rapid diagnosis of mycobacterial lymphadenitis in children, Journal of Clinical Microbiology 42, 2004, 2644–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buergelt C.D., Fowler J.L., Wright P.J. Disseminated avian tuberculosis in a cat, California Veterinarian 10, 1982, 13–15. [Google Scholar]
- Butler W.R., Guthertz L.S. Mycolic acid analysis by high-performance liquid chromatography for identification of Mycobacterium species, Clinical Microbiology Reviews 14, 2001, 704–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caeiro J.P., Iannini P.B. Moxifloxacin (Avelox): a novel fluoroquinolone with a broad spectrum of activity, Expert Review of Anti-infective Therapy 1, 2003, 363–370. [DOI] [PubMed] [Google Scholar]
- Chaisson R.E., Benson C.A., Dube M.P., Heifets L.B., Korvick J.A., Elkin S., Smith T., Craft J.C., Sattler F.R. Clarithromycin therapy for bacteremic Mycobacterium avium complex disease. A randomized, double-blind, dose-ranging study in patients with AIDS. AIDS Clinical Trials Group Protocol 157 Study Team, Annals of Internal Medicine 121, 1994, 905–911. [DOI] [PubMed] [Google Scholar]
- Davies C., Troy G.C. Deep mycotic infections in cats, Journal of the American Animal Hospital Association 32, 1996, 380–391. [DOI] [PubMed] [Google Scholar]
- Deykin A.R., Wigney D.I., Smith J.S., Young B.D. Corneal granuloma caused by Mycobacterium intracellulare in a cat, Australian Veterinary Practitioner 26, 1996, 23–26. [Google Scholar]
- Dietrich U., Arnold P., Guscetti F., Pfyffer G.E., Spiess B. Ocular manifestation of disseminated Mycobacterium simiae infection in a cat, Journal of Small Animal Practice 44, 2003, 121–125. [DOI] [PubMed] [Google Scholar]
- Drolet R. Disseminated tuberculosis caused by Mycobacterium avium in a cat, Journal of the American Veterinary Medical Association 189, 1986, 1336–1337. [PubMed] [Google Scholar]
- Evans L.M., Caylor K.B. Mycobacterial lymphadenitis in a cat, Feline Practice 23, 1995, 14–17. [Google Scholar]
- Foster S.F., Martin P., Davis W., Allan G.S., Mitchell D.H., Malik R. Chronic pneumonia caused by Mycobacterium thermoresistibile in a cat, Journal of Small Animal Practice 40, 1999, 433–438. [DOI] [PubMed] [Google Scholar]
- Grange J.M., Yates M.D., Boughton E. The avian tubercle bacillus and its relatives, Journal of Applied Bacteriology 68, 1990, 411–431. [DOI] [PubMed] [Google Scholar]
- Greene C.E., Gunn-Moore D.A. Mycobacterial infections. Greene C.E. Infectious Diseases of the Dog and Cat, 2nd edn, 1998, WB Saunders: Philadelphia, 313–325. [Google Scholar]
- Greene C.E., Watson A.D.J. Appendix 8, Antimicrobial drug formulary. Greene C.E. Infectious Diseases of the Dog and Cat, 2nd edn, 1998, WB Saunders: Philadelphia, 790–919. [Google Scholar]
- Griffin A., Newton A.L., Aronson L.R., Brown D.C., Hess R.S. Disseminated Mycobacterium avium complex infection following renal transplantation in a cat, Journal of the American Veterinary Medical Association 222, 2003, 1097–1101. [DOI] [PubMed] [Google Scholar]
- Grossman A. Mycobacterial hepatitis associated with long-term steroid therapy, Feline Practice 13, 1983, 37–41. [Google Scholar]
- Gunn-Moore D.A., Jenkins P.A., Lucke V.M. Feline tuberculosis: a literature review and discussion of 19 cases caused by an unusual mycobacterial variant, Veterinary Record 138, 1996, 53–58. [DOI] [PubMed] [Google Scholar]
- Hix J.W., Jones T.C., Karlson A.G. Avian tubercle bacillus infection in the cat, Journal of the American Veterinary Medical Association 138, 1961, 641–647. [PubMed] [Google Scholar]
- Horn B., Forshaw D., Cousins D., Irwin P.J. Disseminated Mycobacterium avium infection in a dog with chronic diarrhoea, Australian Veterinary Journal 78, 2000, 320–325. [DOI] [PubMed] [Google Scholar]
- Horsburgh C.R. The pathophysiology of disseminated Mycobacterium avium complex disease in AIDS, Journal of Infectious Diseases 179, 1999, S461–S465. [DOI] [PubMed] [Google Scholar]
- Hughes M.S., Ball N.W., Beck L.A., Lisle G.W., de Skuce R.A., Neill S.D. Determination of the etiology of presumptive feline leprosy by 16S rRNA gene analysis, Journal of Clinical Microbiology 35, 1997, 2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M.S., Ball N.W., Love D.N., Canfield P.J., Wigney D.I., Dawson D., Davis P.E., Malik R. Disseminated Mycobacterium genavense infection in a FIV-positive cat, Journal of Feline Medicine and Surgery 1, 1999, 23–29. [DOI] [PubMed] [Google Scholar]
- Hughes M.S., James G., Ball N., Scally M., Malik R., Wigney D.I., Martin P., Chen S., Mitchell D., Love D.N. Identification by 16S rRNA gene analyses of a potential novel mycobacterial species as an etiological agent of canine leproid granuloma syndrome, Journal of Clinical Microbiology 38, 2000, 953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M.S., James G., Taylor M.J., McCarroll J., Neill S.D., Chen S.C.A., Mitchell D.H., Love D.N., Malik R. PCR studies of feline leprosy cases, Journal of Feline Medicine and Surgery 6, 2004, 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan H.L., Cohn L.A., Armstrong P.J. Disseminated Mycobacterium avium complex infection in three Siamese cats, Journal of the American Veterinary Medical Association 204, 1994, 90–93. [PubMed] [Google Scholar]
- Kaufman A.C., Greene C.E., Rakich P.M., Weigner D.D. Treatment of localized Mycobacterium avium complex infection with clofazimine and doxycycline in a cat, Journal of the American Veterinary Medical Association 207, 1995, 457–459. [PubMed] [Google Scholar]
- Knippel A., Hetzel U., Baumgartner W. Disseminated Mycobacterium avium-intracellulare infection in a Persian cat, Journal of Veterinary Medicine Series B Infectious Diseases and Veterinary Public Health 51, 2004, 464–466. [DOI] [PubMed] [Google Scholar]
- Latimer K.S., Jameson P.H., Crowell W.A., Duncan J.R., Currin K.P. Disseminated Mycobacterium avium complex infection in a cat: presumptive diagnosis by blood smear, Veterinary Clinical Pathology 26, 1997, 85–89. [DOI] [PubMed] [Google Scholar]
- McCauley M., Atwell R.B., Sutton R.H., Lumsden J.S. Unguided bronchoalveolar lavage techniques and residual effects in dogs, Australian Veterinary Journal 76, 1998, 161–165. [DOI] [PubMed] [Google Scholar]
- MacWilliams P.S., Whitley N., Moore F. Lymphadenitis and peritonitis caused by Mycobacterium xenopi in a cat, Veterinary Clinical Pathology 27, 1998, 50–53. [DOI] [PubMed] [Google Scholar]
- Malik R., Gabor L., Martin P., Mitchell D.H., Dawson D.J. Subcutaneous granuloma caused by Mycobacterium avium complex infection in a cat, Australian Veterinary Journal 76, 1998, 604–607. [DOI] [PubMed] [Google Scholar]
- Malik R., Wigney D.I., Dawson D., Martin P., Hunt G.B., Love D.N. Infections on the subcutis and skin of cats with rapidly growing mycobacteria: a review of microbiological and clinical findings, Journal of Feline Medicine and Surgery 2, 2000, 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R., Hughes M.S., Love D.N. Nontuberculous mycobacterial disease. August J.R. Consultations in Feline Internal Medicine Vol 4, 2001, WB Saunders: Philadelphia, 221–232. [Google Scholar]
- Malik R., Hughes M.S., James G., Martin P., Wigney D.I., Canfield P.J., Chen S.C.A., Mitchell D.H., Love D.N. Feline leprosy: two different clinical syndromes, Journal of Feline Medicine and Surgery 4, 2002, 43–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur H. Recommendations on prophylaxis and therapy for disseminated Mycobacterium avium disease complex in patients infected with the human immunodeficiency virus, The New England Journal of Medicine 329, 1993, 898–904. [DOI] [PubMed] [Google Scholar]
- Matthews J.A., Liggitt H.D. Disseminated mycobacteriosis in a cat, Journal of the American Veterinary Medical Association 183, 1983, 701–702. [PubMed] [Google Scholar]
- Mealey K.L., Willard M.D., Nagode L.A., Helman R.G. Hypercalcemia associated with granulomatous disease in a cat, Journal of the American Veterinary Medical Association 215, 1999, 959–962. [PubMed] [Google Scholar]
- Meissner G., Schroder K.H., Amadio G.E., Anz W., Chaparas S., Engel H.W.B., Jenkins P.A., Kappler W., Kleeberg H.H., Kubala E., Kubin M., Lauterbach D., Lind A., Magnusson M., Mikova Z., Pattyn S.R., Schaefer W.B., Stanford J.L., Tsukamura M., Wayne L.G., Willers I., Wolinsky E. A co-operative numerical analysis of nonscoto- and nonphotochromagenic slowly growing mycobacteria, Journal of General Microbiology 83, 1974, 207–235. [DOI] [PubMed] [Google Scholar]
- Michaud A.J. The use of clofazimine as treatment for Mycobacterium fortuitum in a cat, Feline Practice 22, 1994, 7–9. [Google Scholar]
- Montali R.J., Bush M., Cromie R., Holland S.M., Maslow J.N., Worley M., Witebsky F.G., Phillips T.M. Primary Mycobacterium avium complex infections correlate with lowered cellular immune reactivity in Matschie's tree kangaroos (Dendrolagus matschiei), Journal of Infectious Diseases 178, 1998, 1719–1725. [DOI] [PubMed] [Google Scholar]
- Morfitt D.C., Matthews J.A., Thoen C.O., Kluge J.P. Disseminated Mycobacterium avium serotype 1 infection in a seven-month-old cat, Journal of Veterinary Diagnostic Investigations 1, 1989, 354–356. [DOI] [PubMed] [Google Scholar]
- Naughton J.F., Mealey K.L., Wardrop K.J., Oaks J.L., Bradway D.S. Systemic Mycobacterium avium infection in a dog diagnosed by polymerase chain reaction analysis of buffy coat, Journal of the American Animal Hospital Association 41, 2005, 128–132. [DOI] [PubMed] [Google Scholar]
- Perkins P.C., Grindem C.B., Levy J.K. What is your diagnosis? An 11-year-old domestic longhaired cat with anaemia, Veterinary Clinical Pathology 24 (77), 1995, 97–98. [DOI] [PubMed] [Google Scholar]
- Pinson D.M., Tucker L. Mycobacteriosis in a cat, Veterinary Medicine 93, 1998, 440–441. [Google Scholar]
- Ramasoota P., Chansiripornchai N., Kallenius G., Hoffner S.E., Svenson S.B. Comparison of Mycobacterium avium complex (MAC) strains from pigs and humans in Sweden by random amplified polymorphic DNA (RAPD) using standardized reagents, Veterinary Microbiology 78, 2001, 251–259. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Labrousse V. Extracellular and intracellular activities of clarithromycin used alone and in association with ethambutol and rifampin against Mycobacterium avium complex, Antimicrobial Agents and Chemotherapy 35, 1991, 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remus N., Reichenbach J., Picard C., Rietschel C., Wood P., Lammas D., Kumararatne D.S., Casanova J.L. Impaired interferon gamma-mediated immunity and susceptibility to mycobacterial infection in childhood, Pediatric Research 50, 2001, 8–13. [DOI] [PubMed] [Google Scholar]
- Sieber-Ruckstuhl NS, Sessions JK, Sanchez S, Greene CE, Successful treatment of disseminated Mycobacterium avium infection in a 5-year-old Abyssinian. Journal of the American Veterinary Medical Association, in preparation.
- Sifford M., Bates J.H. Host determinants of susceptibility to Mycobacterum tuberculosis , Seminars in Respiratory Infections 6, 1991, 44–50. [PubMed] [Google Scholar]
- Studdert V.P., Hughes K.L. Treatment of opportunistic mycobacterial infections with enrofloxacin in cats, Journal of the American Veterinary Medical Association 201, 1992, 1388–1390. [PubMed] [Google Scholar]
- Thompson J.P. Disorders of the immune system. Scherding R.G. The Cat: Diseases and Clinical Management, 2nd edn, 1994, Churchill Livingstone Inc: NYC, 647–670. [Google Scholar]
- Thoresen O.F., Saxegaard F. Comparative use of DNA probes for Mycobacterium avium and Mycobacterium intracellulare and serotyping for identification and characterization of animal isolates of the M avium complex, Veterinary Microbiology 34, 1993, 83–88. [DOI] [PubMed] [Google Scholar]
- Tomioka H., Sano C., Sato K., Shimizu T. Antimicrobial activities of clarithromycin, gatifloxacin and sitafloxacin, in combination with various antimycobacterial drugs against extracellular and intramacrophage Mycobacterium avium complex, International Journal of Antimicrobial Agents 19, 2002, 139–145. [DOI] [PubMed] [Google Scholar]
- van Dongen A.M., Wagenaar J.A., Kraus H.S., Noordhoek G.T., Eger A., Ottenschot T.R.F. Atypical mycobacteriosis in a cat, Veterinary Quarterly 18, 1996, S47. [DOI] [PubMed] [Google Scholar]
- Wayne L.G., Good R.C., Krichevsky M.I., Beam R.E., Blacklock Z., Chaparas S.D., Dawson D., Froman S., Gross W., Hawkins J., Jenkins P.A., Juhlin I., Kappler W., Kleeberg H.H., Krasnow I., Lefford M.J., Mankiewicz E., McDurmont C., Meissner G., Morgan P., Nel E.E., Pattyn S.R., Portaels F., Richards P.A., Rusch S., Schroder K.H., Silcox V.A., Szabo I., Tsukamura M., Vergmann B. First report of the cooperative, open-ended study of slowly growing mycobacteria by the International Working Group on Mycobacterial Taxonomy, International Journal of Systematic Bacteriology 31, 1981, 1–20. [DOI] [PubMed] [Google Scholar]
- Wolff A. Tuberculosis in a domestic cat, Veterinary Medicine and Small Animal Clinician 61, 1966, 553. [PubMed] [Google Scholar]