Abstract
Background:
Laryngoscopy and tracheal intubation lead to sympathetic stimulation resulting in hemodynamic fluctuations. We compared local anesthetic ropivacaine 0.75% with alpha agonist dexmedetomidine through ultrasonic nebulization for direct local action of the drug in the airway.
Methods:
In our randomized study, 180 patients were prospectively assigned to three groups of 60 each: group R (0.75%), group D (1 microgram/kg), and group C (control). The primary objective was to determine whether nebulized ropivacaine or nebulized dexmedetomidine can cause a reduction in stress response to laryngoscopy and intubation. The secondary objectives were to compare the hemodynamic parameters at extubation, cough response at extubation, and postoperative sore throat.
Results:
A total of 165 patients were analyzed. Demographically, all the groups were similar. Group R and group D were found to significantly attenuate the heart rate (HR) at intubation and extubation when compared to group C (P < 0.05). A significant reduction in mean arterial pressure (MAP) was seen (P < 0.05; group D: 90 ± 18.4 mmHg, group C: 99.5 ± 15.9 mmHg, group R: 92.4 ± 16.1 mmHg). There was a significant reduction in cough response in both groups in comparison with group C at 0 minutes (P value; group C vs group D: <.0001; group C vs group R:.01) and 5 minutes (P value; group C vs group D: <.0001; group C vs group R: <.0001).
Conclusion:
Preinduction topical use of ropivacaine or dexmedetomidine, through the nebulization route, effectively attenuated the pressor responses when compared to placebo.
Keywords: Airway management, cough, dexmedetomidine, perioperative care, ropivacaine, sore throat, tracheal extubation
Introduction
The larynx is a richly innervated structure with abundant neurovascular supply. Laryngoscopy and tracheal intubation are generally associated with pressure on the larynx, which results in the activation of somatovisceral reflexes. Sympathoadrenal activation leads to the release of catecholamines, which results in a rise in hemodynamics.[1] The duration of the intubation and the force exerted during the procedure directly affect the catecholamine response. It starts within 5–10 seconds, peaking at 1–2 minutes, and finally returning to its baseline readings in the next 5 minutes.[2] Although this is a transient response with an average rise in systolic blood pressure (SBP) of 25–30 mmHg, and most healthy patients tolerate it without any untoward effect, it may prove to be extremely deleterious in patients with cerebrovascular or cardiovascular diseases.[3] It has been associated with the precipitation of cardiac ischemia, cerebral stroke, pulmonary edema, etc.[4]
Various preemptive measures are used to attenuate this response, mainly addressed by intravenous or local agents. Most commonly employed are α2 agonists, opioids, beta-blockers, and local anesthetics.[5] All of them are associated with various systemic side effects such as opioids with reduced respiratory drive, sedation, and postoperative nausea and vomiting (PONV) and selective alpha 2 agonists such as dexmedetomidine with hypotension and bradycardia. Most of these are used preemptively, but very few are administered through the nebulization route. Direct instillation through nebulization is a new area with lesser systemic effects, which needs to be explored.
Ropivacaine, a long-acting local anesthetic, had been widely used through various routes for anesthesia and postoperative analgesia. Topical administration (maybe through nebulization, nerve blocks, intratracheal instillation, or atomization) has piqued the interest of recent researchers. In various studies, it had been used through the nebulization route, where it had shown promising results. Thangavelu et al. reported attenuation of hemodynamic response at intubation when it was administered through the nebulization route at 0.25% concentration.[3] Administration of local anesthetic ropivacaine at intubation also attenuates the hemodynamic fluctuation and cough response at extubation due to a longer duration of action as compared to lignocaine. Fang P et al. also recommended topical instillation for ropivacaine (0.75%) for reduced stress response at intubation and extubation.[6]
Dexmedetomidine, an alpha 2 agonist, had been widely studied through the intravenous route where it has repeatedly proven itself to suppress the hemodynamic stress response, but systemic side effects such as hypotension and bradycardia are commonly associated, which may be troublesome.[7] However, the recent literature has stated that the nebulization route has better bioavailability: nasal mucosa—65% and buccal mucosa—82%. Nebulized dexmedetomidine has been reported with a very short distribution half-life (6 minutes) and elimination half-life (2 hours) without being associated with systemic side effects.[8] It has been widely used as a premedication in pediatric patients for procedural sedation and premedication, where it has shown promising results.[9,10,11,12] Hussain et al. have reported in the literature that dexmedetomidine when administered through the nebulization route had reduced stress response to laryngoscopy and intubation.[13] Thomas D et al. also reported that nebulized dexmedetomidine can attenuate the postoperative sore throat in patients.[14]
To the best of our knowledge, there were no documented studies comparing the effect of ropivacaine (0.75%) with dexmedetomidine through the nebulization route to reduce hemodynamic response during intubation in patients with general anesthesia (GA).
Thus, the primary aim of this study was to compare the hemodynamic response of 0.75% nebulized ropivacaine with nebulized dexmedetomidine (1 µg/kg) and with nebulized saline during intubation in patients who underwent surgeries under GA.
Materials and Methods
Study design
It was a prospective randomized, double-blinded clinical trial.
Study participants
All adult patients of ages 18–60 years, with American Society of Anesthesiologists grade I or II of both genders, and who gave consent were included. These were posted for elective surgery (duration <120 minutes) requiring GA.
Study approval and trial registration
The study was approved by the Institutional Ethics Committee (AIMS/IEC/15/2022), and written informed consent was obtained from each subject. Prospective registration of the study was conducted in the Clinical Trials Registry of India (CTRI) (trial registration number: CTRI/2022/07/044029). This study followed the ethical principles for medical research (for human subjects in accordance with the Helsinki Declaration of 2013).
Exclusion criteria
We excluded the following patients from the study:
Patient with a history of sore throat or upper respiratory tract infection
Hemodynamically unstable patients
Emergency patients
Patients with a history of allergy to local anesthetics
Patents with a history of allergy to dexmedetomidine
Patients with a history of cardiac, liver, or renal disorders.
Patient with a difficult airway where the anticipated duration of laryngoscopy exceeded 15 seconds.
Randomization and allocation concealment
A total of 180 patients planned for elective surgery under GA were randomly allocated to three groups of 60 (group D, group R, and group C) patients each using a computer-generated random code and the allotted code secured in a coded opaque sealed envelope, which was opened in the preoperative procedure area on the day of the surgery. The procedure was performed 15 minutes before the anticipated intubation.
Group R received ultrasonic nebulization with 0.75% ropivacaine (5 ml).
Group D received ultrasonic nebulization with dexmedetomidine (1 µg/kg) with normal saline (5 ml).
Group C received ultrasonic nebulization with normal saline (5 ml).
Preparation of the patient
All the patients were evaluated in the preoperative clinic, one day before the surgery. A complete physical examination was conducted to assess the fitness for the tentative surgical procedure under GA.
A preoperative airway evaluation was performed on all the patients. Routine investigations were performed on all the patients. Any other relevant special investigation whenever necessary was ordered. Patients were advised to take a tablet of alprazolam 0.25 mg the night before surgery and 6.30 AM and a tablet of pantoprazole 40 mg on the day of surgery and were kept fasting for 6 hours (solids) and 2 hours (plain clear water).
The consent was taken one day prior, by either the principal investigator or coinvestigator after explaining the procedure. The patient was given an information sheet (English and local language) for an explanation, and any query was answered by the investigator. The consent form was signed in the presence of two unrelated witnesses. Any query along the procedure was addressed.
Nebulization procedure
The primary anesthesiologist with at least 3 years of experience blinded to the assignments prepared the randomized drug (as per the randomized generated list) of patients in the three groups. Nebulization was performed with the help of an ultrasonic nebulizer (Yuwell 402AI™ Ultrasonic Nebulizer), which created a fine mist. The nebulization was performed until the entire volume was dispersed (5–7 minutes). The procedure was stopped until there was no further mist generation. One of the investigators oversaw the entire nebulization procedure and was authorized to intervene whether any side effects such as bradycardia, increased sedation, and coughing were reported. If so, the procedure was stopped and the patient was treated as per the institutional policy.
Anesthesia protocol
Conduct of anesthesia
After arrival in the operative room, monitors as per American Society of Anesthesiologists Classification (ASA) guidelines were attached and baseline parameters (heart rate (HR), noninvasive blood pressure (NIBP), continuous electrocardiogram (ECG), end-tidal carbon dioxide (EtCO2), and arterial oxygen saturation (SpO2)) were monitored. An intravenous line was secured with an 18 G cannula, and all patients received normal saline infusion at a rate of 10 ml/kg/hr.
The primary blinded anesthesiologist who had prepared the drug was not involved further. The second anesthesiologist administered the anesthesia as per the institutional protocol and performed the tracheal intubation of the patient. A third anesthesiologist recorded the required parameters.
All patients will be premedicated with injection (Inj.) of glycopyrrolate 0.2 mg and midazolam 0.04 mg/kg, 5 minutes before induction. Anesthesia will be induced in the supine position with the head on a standard pillow of 7 cm in height. After preoxygenation for three minutes, anesthesia will be induced using morphine 0.1 mg/kg and propofol 1.5 to 2.5 mg/kg. After checking for the ability to achieve adequate mask ventilation, vecuronium 0.1 mg/kg will be used to facilitate muscle relaxation. Assisted mask ventilation with 1% isoflurane in oxygen will be performed using a circle system (Draeger Fabius Plus) for 4 minutes. The inner diameter (ID) of the endotracheal tube used will be 7.0–7.5 mm for female patients and 7.5–8.0 mm for male patients. Intubation will be performed by direct laryngoscopy. The duration of laryngoscopy will be kept at <15 seconds. If in any case, a second attempt or duration exceeds 15 seconds, the patient will be excluded from the study. After successful intubation, mechanical ventilation (Tidal volume: 6–8 ml/kg, respiratory rate: 10–12 breaths/min, expiration to inspiration ratio = 2:1) will be started, and the EtCO2 pressure shall be maintained at 35–40 mmHg. Anesthesia will be maintained with nitrous oxide and oxygen mixture in the ratio of 2:1 and isoflurane (1 mean alveolar concentration), and intravenous muscle relaxants will be given every 30–40 min to maintain the neuromuscular paralysis. At the end of surgery, 15 mg/kg paracetamol and 0.1 mg/kg ondansetron intravenously will be administered for postoperative pain and emesis, respectively.
The oropharynx shall be suctioned, the inhalational agent stopped, and the patient will be administered 100% oxygen. After the return of spontaneous respiration, the neuromuscular blockade will be reversed with 0.05 mg/kg of neostigmine and 0.01 mg/kg of inj. of glycopyrrolate. The trachea shall be extubated when the patient demonstrates the ability to follow verbal commands or shows purposeful movements in addition to resumption of regular spontaneous respiration.
Patients were kept in the postoperative care unit for an additional 3 h and discharged to the ward once they met the criteria for discharge.
Primary aim and outcome parameters
The primary aim of the study was to compare the hemodynamic response with nebulized ropivacaine 0.75% with nebulized dexmedetomidine ((1 µg/kg) and with normal saline during intubation, and accordingly, the parameters were measured at various time points:
T0—baseline before the procedure of nebulization
T1—after nebulization
T2—before tracheal intubation
T3—after tracheal intubation
T4—5 minutes after tracheal intubation
T5—at the closure of skin incision
T6—immediately after tracheal extubation
T7—5 minutes after tracheal extubation
Secondary aims and outcome parameters
To compare the hemodynamic parameters at extubation.
To compare the cough response at extubation and the incidence rate of postoperative sore throat.
The severity of the cough at the time of extubation was assessed with the following grade:
None—no cough
Mild—a single cough
Moderate—cough of the duration of less than 5 seconds
Severe—continuous coughing of the duration of more than 5 seconds
Postoperative sore throat at various interval points (0, 2, 4, 6, 12, and 24 hr) was assessed with the following grade:
0—no complaint of any sore throat
1—(mild) complains of sore throat only when asked
2—(moderate) patient complains of sore throat even without asking
3—(severe) patient complaints of change in voice or hoarseness and pain in the throat
Any adverse event in the perioperative period will be managed as per the institutional policy.
Sample size calculation
The sample size calculation was based on a study by Sale H K et al.,[7] where they compared intravenous lignocaine with dexmedetomidine. They studied the effect of the drugs on blunting the intubation response. The effect size was calculated by comparing the difference in the mean arterial pressures from baseline (91.00 ± 7.80) and 1 minute after the intubation (80.50 ± 7.09). With a power of 90% and alpha error of 0.05, the calculation of sample size came about to be 147 (49 patients in one group) and a total of 180 patients were to be included in the study, including those who withdraw/dropouts due to various reasons from the study.
All data were expressed as mean (standard deviation (SD)), median (range), or number (proportion, %). Analysis was performed with Statistical Package for the Social Sciences (SPSS) version 25.0 (SPSS Inc., Chicago, USA). An unpaired two-tailed Student's t-test was used to compare normally distributed continuous variables. An analysis of variance was utilized to find the significance of hemodynamic parameters among all three groups at various time intervals. This was followed by a post hoc Tukey's test. The Chi-square test or Fisher's exact test was used for categorical data. A P value of < 0.05 was documented as statistically significant.
Results
A total of 180 patients were enrolled in the study over a six-month period (study start date: July 15, 2022; end date: January 14, 2023). We encountered attrition of 15 patients due to various factors (unanticipated difficult intubation and prolonged laryngoscopy); hence, 165 patients were analyzed statistically. The demographic variables of the subjects are presented in Table 1. Surgeries (duration of <120 minutes) were from various specialities (general surgery, orthopedics, ear, nose, and throat (ENT), and gynecology). All three groups were comparable demographically with respect to all variables (age, height, weight, gender, and ASA PS grade), duration of anesthesia, or duration of surgery (P > 0.05 each).
Table 1.
Demographic characteristics
| Group D (n=52) | Group C (n=59) | Group R (n=54) | |
|---|---|---|---|
| Age (in years) | 37.5±11.2 | 37.7±10.2 | 35.3±11.5 |
| Weight (in kg) | 66.3±14.4 | 71.4±12.8 | 70.7±14.8 |
| Height (in cm) | 163±5.04 | 160±7.61 | 158±14.5 |
| Gender (M/F) | 11/41 | 17/42 | 27/27 |
| ASA PS (I/II) | 38/14 | 48/11 | 45/9 |
| Duration of Surgery (in min) | 61.6±29.9 | 70.9±24 | 81.6±25.3 |
| Duration of Anaesthesia (in min) | 68.9±30.3 | 80.6±24.3 | 90.6±24.1 |
Values are in mean±SD. ASA PS: American Society of Anaesthesiologists Physical Status
The hemodynamic parameters were compared between the three groups at different time points. Both drugs (dexmedetomidine and ropivacaine) were found to reduce the HR and SBP significantly at intubation and extubation [Table 2]. A statistically significant difference (P > 0.05) in HR was noted between the three groups at intubation (T3) and extubation (T6) [Figure 1].
Table 2.
Table showing the hemodynamic parameters
| Group D | Group C | Group R | ANOVA P (Tukey’s test) | Pair-wise comparison (P) |
|||
|---|---|---|---|---|---|---|---|
| GD vs GC | GD vs GR | GC vs GR | |||||
| Mean arterial pressure (MAP) | |||||||
| T0 | 94.1±10.2 | 92.3±12.1 | 96.9±13.5 | 0.12 | 0.70 | 0.45 | 0.10 |
| T1 | 91.1±10.5 | 89.8±13.7 | 94.2±12.7 | 0.16 | 0.84 | 0.41 | 0.14 |
| T2 | 89.9±12.2 | 90.7±14.3 | 85.4±16.3 | 0.11 | 0.94 | 0.25 | 0.12 |
| T3 | 90±18.4 | 99.5±15.9 | 92.4±16.1 | 0.009 | 0.01 | 0.74 | 0.07 |
| T4 | 95±17.2 | 92.6±12.6 | 88.7±12 | 0.06 | 0.62 | 0.05 | 0.31 |
| T5 | 89.9±14.2 | 94.7±11.5 | 90.4±12.4 | 0.09 | 0.11 | 0.97 | 0.18 |
| T6 | 103±14.5 | 109±12.5 | 10±17 | 0.03 | 0.06 | 0.99 | 0.06 |
| T7 | 97.9±10.8 | 99.2±11.9 | 96.1±12.7 | 0.39 | 0.84 | 0.72 | 0.36 |
Values are in mean±SD. T0 - Before nebulization, T1 - Immediately after nebulization, T2 - Before intubation, T3 - Immediately after intubation, T4 - 5 mins after intubation, T5 - At the end of surgery (closure of skin incision), T6 - Immediately after extubation, and T7 - 5 min after extubation
Figure 1.
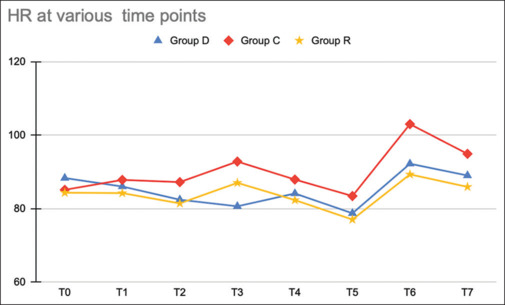
Line diagram showing heart rate among the three groups at various time points (T0—before nebulization, T1—immediately after nebulization, T2—before intubation, T3*—immediately after intubation, T4—5 mins after intubation, T5—at the end of surgery (closure of skin incision), T6*—immediately after extubation, and T7*—5 minutes after extubation). (P value was significant at T3 (P < 0.0001), T6 (P < 0.0001), and T7 (P < 0.005))
In group-wise comparison, both groups had a statistically significant difference in SBP at intubation when compared with group C. Regarding diastolic blood pressure (DBP), only group D showed a significant decrease at intubation in comparison with the other two groups [Figure 2]. In comparison between the three groups regarding mean arterial pressure (MAP), though group-wise comparison did not show any significant result, overall it was significant as per the analysis of variance (ANOVA). There was a significant decrease in cough response in both groups in comparison with group C at 0 minutes (P value group C vs group D: <.0001; group C vs group R:.01) and 5 minutes (P value group C vs group D: <.0001; group C vs group R: <.0001). Similar results for post operative sore throat (POST) were observed at 0 and 2 hours (P value < .05 in group D and group R in comparison with group C) [Table 3].
Figure 2.
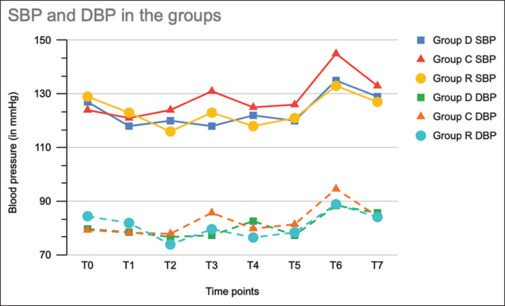
Line diagram showing blood pressure: systolic blood pressure (SBP) and diastolic blood pressure (DBP) among the three groups at various time points. (T0—before nebulization, T1—immediately after nebulization, T2—before intubation, T3*—immediately after intubation, T4—5 mins after intubation, T5—at the end of surgery (closure of skin incision), T6*—immediately after extubation, and T7—5 minutes after extubation). (SBP P value was significant at T3 (P 0.002) and T6 (P < 0.0001); DBP P value was significant at T3 (P 0.02) and T6 (P 0.05))
Table 3.
Peri extubation cough and post-operative sore throat (POST)
| Group D | Group C | Group R | |
|---|---|---|---|
| Cough (None/Mild/Moderate/Severe) | |||
| 0 min | 39/12/1/0 | 21/31/7/0 | 32/20/2/0 |
| 5 min | 49/3/0/0 | 40/18/1/0 | 53/1/0/0 |
| 30 min | 52/0/0/0 | 53/6/0/0 | 54/0/0/0 |
| POST (Grade 0/1/2/3) | |||
| 0 h | 44/7/1/0 | 27/30/2/0 | 46/8/0/0 |
| 2 h | 49/3/0/0 | 36/23/0/0 | 53/1/0/0 |
| 4 h | 51/1/0/0 | 50/9/0/0 | 54/0/0/0 |
| 6 h | 52/0/0/0 | 57/2/0/0 | 54/0/0/0 |
| 12 h | 52/0/0/0 | 59/0/0/0 | 54/0/0/0 |
| 24 h | 52/0/0/0 | 59/0/0/0 | 54/0/0/0 |
In group C and group D, none of the patients had cough at 30 minutes in comparison with group C. None of the patients had POST group D after 6 hours and group R at 4 hours. There was one patient who developed nausea after nebulization with ropivacaine; except for this, we did not encounter any adverse event.
Discussion
Laryngoscopy and tracheal intubation are often associated with a sudden rise in catecholamine levels resulting in an increase in hemodynamic parameters. A similar response is seen at extubation of the trachea. Various factors are responsible for hemodynamic response following laryngoscopy and intubation, which include epiglottis elevation, displacement of the tongue, and increased duration in laryngoscopy. The resultant rise in blood pressure and HR may adversely affect patients with myocardial infarction, cardiac arrhythmias, internal hemorrhage, etc. Various methods and drugs have been mentioned in the literature, which attenuates the rise. α2 agonists, opioids, local anesthetics (lignocaine), and beta-blockers are given prophylactically at the time of both intubation and extubation to obtund the hemodynamic pressor response, but most of the drugs are associated with unwanted systemic side effects, which delay the process of the extubation, especially due to sedation.[15,16] Various local anesthetics such as lignocaine were used systemically to obtund the pressor response where statistically significant results were seen.[17] Later on, topical application (spray, nebulization, and lozenges) of local anesthetics also showed promising results. Lignocaine, owing to its short duration of action, did not show any effect at the time of extubation.[3] In view of the short duration of action of lignocaine, ropivacaine was explored in various studies through local route to attenuate the pressor response. Various studies found a significant reduction in pressor response both at intubation and at extubation.[3,18]
The effect of ropivacaine is mainly because of its action on sympathetic nerves in the airway, which is blocked by it.[19] Concentrations of ropivacaine varying from 0.25 to 0.75% have been studied with promising results. In our study, group R had a statistically significant reduction in HR and MAP at intubation and extubation. Similar results were documented in the study published by Gao et al. where the patients received ropivacaine through transcricoid Inj. before induction.[18] The long duration of these newer local anesthetics also provided attenuation of pressor response at extubation. Meng et al. showed in the randomized trial a decrease in HR and MAP significantly in hypertensive patients undergoing surgery,[5] whereas in Thangavelu et al., ropivacaine had a significant reduction at induction but not extubation when compared with lignocaine.[3] This could have been due to their lower concentration of ropivacaine (0.25%).
Dexmedetomidine, an alpha-2A receptor agonist, acts on the receptors, which are located in various regions of the brain (locus coeruleus and noradrenergic nuclei in upper brain stem), and it leads to inhibition of noradrenaline release.[20] Due to its varied effects, which include sedation, hypnosis, anxiolysis, sympatholysis, and analgesia, it has made an important position in every anesthesiologist's armamentarium. Although it is associated with bradycardia and hypotension, it is easily manageable in controlled settings. It has been extensively studied (when given by intravenous route) to attenuate the hemodynamic pressor response after intubation, but very few studies have concluded on its use through other routes.[21,22] Nebulization as a novel route was considered as an alternative recently. Through this route, the bioavailability of drug is better (nasal mucosa—65% and buccal mucosa—82%).[11] Dexmedetomidine has been used with many other drugs through nebulization such as ketamine, midazolam, and lignocaine, especially in the pediatric population, mainly as a premedication, but very few studies have seen its effect on pressor response.[23,24,25]
Misra et al. published that dexmedetomidine through the nebulization route leads to attenuation of the increase in HR but not SBP when measured at different time points.[26] The dose of propofol was also significantly reduced.
In our study, group D received nebulized dexmedetomidine and showed a statistically significant reduction in hemodynamic parameters (HR and mean blood pressure) after intubation was seen. We also observed a decrease in SBP and DBP as compared to the control group. A study by Kumar et al. also had a similar conclusion that nebulized dexmedetomidine led to a decrease in the hemodynamic response to laryngoscopy and intubation without leading to any adverse effects.[8] Both studies only concluded the results after intubation, and none of them compared the data at the time of extubation. We observed a significant reduction in the hemodynamic parameters at extubation as well. As all surgeries took less than 2 hours, the action of the drug was still present till the time of extubation.
Hussain et al. also documented the effect of dexmedetomidine through nebulization on pressor response, but the dose of dexmedetomidine (2 mics/kg) was twice as that included in our study.[13] Shrivastava et al. published in their study that nebulized dexmedetomidine at a dose of 1 mics/kg led to attenuation in the hemodynamic response to laryngoscopy and intubation, while avoiding hypotension, bradycardia, and sedation, which was similar to our study.[20]
In our prospective trial, we also found a statistically significant decrease in pressor response both at intubation and at extubation. No major side effects were observed from this procedure.
Both interventions were also associated with a significant reduction in the incidence of POST and cough in the postoperative period, which is usually seen after surgeries performed under GA.
In our study, we also concluded that there was a significant reduction in the incidence of POST and cough in the postoperative period, and very few studies have explored this outcome. The incidence rate of postoperative sore throat after tracheal intubation is very variable, ranging from 21 to 65%. It is ranked eighth place in the adverse events, which are documented in the postoperative period.[27] It occurs as a result of pressure injury to the pharyngeal and tracheal mucosal layer, which may incite an aseptic inflammatory reaction in the tracheal mucosa. The time period of 4–6 hours after extubation was the most common time period for complaint of POST. Previous reports have documented the favorable effects of dexmedetomidine. It leads to bronchodilatation by relaxing the smooth muscles due to its effect on peripheral α2A adrenoreceptors directly; thus, we sought to investigate whether there is an effect of dexmedetomidine or ropivacaine on the incidence of postoperative sore throat.
Thomas et al. studied the nebulized dexmedetomidine with nebulized ketamine and found it to be a better alternative to ketamine in the prevention of POST.[14] Although Misra et al. did not find any beneficial effect on POST as contrary to our study, it may be due to the long duration of surgeries (>2 hours) and the procedure of nebulization was performed 30 minutes before intubation.[26] By this time, dexmedetomidine may have been eliminated. In our study, ropivacaine was also a good alternative to prevent POST preemptively. Not many studies have explored this arena.
Coughing is also an unanticipated adverse event, which is troublesome both for the patient and for anesthesiologists. The activation of mechanoreceptors due to positive pressure and tracheal tube provokes the coughing response.[28,29]
In our study, group R (ropivacaine) was also effective in a reduction in the incidence of cough and POST. Thangavelu et al. reported only 40% incidence of cough at extubation as compared to 92% in the saline group and 88% in the lignocaine group, which was statistically significant and similar to our study.[3] Ropivacaine has not been extensively researched for its effect on coughing and POST.
Niu et al. studied the effect of dexmedetomidine and ropivacaine individually, and in combination, and they found a significant reduction in the incidence of POST, especially in the group where they used the combination.[30]
Although the mechanism of action of both interventional drugs is different, both have been documented to prevent the hemodynamic pressor response. More research in this area is warranted, maybe even if any additive action is there or not.
In our study, both groups have beneficial effect on POST and cough, especially at extubation, and none of the patients in the intervention groups had POST after 4 hours. In some cases, where it is of utmost importance to avoid any cough response such as neurosurgery and cardiac surgery, these drugs can be hugely helpful because they can attenuate the pressor response and decreased incidence of POST and cough.
Limitations
Our study had many limitations. We included cases with laryngoscopy time <15 seconds, which in itself prevents the pressor response, and we did not explore cases where laryngoscopy time exceeded 15 seconds. We did not estimate the changes in intracuff pressure, which could have adversely affected the incidence of POST. ASA I–II status patients were only enrolled with ages 18–60 years. Elderly critical patients were not included in the study. All the patients received opioid-based anesthesia, which in itself attenuates the pressor response, but it was common among all groups. Although we used an ultrasonic nebulizer, which may not be present at every center, further research is warranted if the wall-mounted oxygen-driven nebulized mask is used.
Conclusion
Preinduction ropivacaine and dexmedetomidine, both when used topically for airway anesthesia through the route of nebulization, effectively attenuated both intubation and extubation responses in comparison with placebo. However, there was no statistically significant difference between the two drugs, ropivacaine and dexmedetomidine, at any point. Both are good alternatives to be used to prevent the hemodynamic pressor response, POST, and cough.
Main Points:
The study mainly focuses on the effect of nebulized ropivacaine (0.75%) and nebulized dexmedetomidine on the hemodynamic pressor response at intubation and extubation. Both drugs were effective in significantly reducing the hemodynamic pressor response when compared with a placebo.
Both drugs were also effective in reducing the post-extubation cough, which was statistically significant.
The incidence of postoperative sore throat was also noted to be less in both groups.
Both ropivacaine and dexmedetomidine are good alternatives, when given through the nebulization route, to obtund the hemodynamic pressor response at both intubation and extubation (long duration of action) while avoiding the systemic side effects.
These can be very helpful, especially in cases where any hemodynamic change can be deleterious to the patient.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chandramohan V, Natarajan R, Hiremath VR. Comparative study of hemodynamic responses during laryngoscopy and endotracheal intubation with dexmedetomidine and esmolol. Asian J Med Sci. 2022;13:125–31.. [Google Scholar]
- 2.Kovac AL. Controlling the hemodynamic response to laryngoscopy and endotracheal intubation. J Clin Anesth. 1996;8:63–79. doi: 10.1016/0952-8180(95)00147-6. [DOI] [PubMed] [Google Scholar]
- 3.Thangavelu R, Ventakesh R, Ravichandran K. Comparison of effect of airway nebulization with lignocaine 2% versus ropivacaine 0.25% on intubation and extubation response in patients undergoing surgery under general anaesthesia: A randomized double-blind clinical trial. Anaesth Essays Res. 2018;12:338–43. doi: 10.4103/aer.AER_83_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aouad MT, Al-Alami AA, Nasr VG, Souki FG, Zbeidy RA, Siddik-Sayyid SM, et al. The effect of low-dose remifentanil on responses to the endotracheal tube during emergence from general anesthesia. Anesth Analg. 2009;108:1157–60. doi: 10.1213/ane.0b013e31819b03d8. [DOI] [PubMed] [Google Scholar]
- 5.Meng YF, Cui GX, Gao W, Li ZW. Local airway anesthesia attenuates hemodynamic responses to intubation and extubation in hypertensive surgical patients. Med Sci Monit. 2014;20:1518–24. doi: 10.12659/MSM.890703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang P, Zong Z, Lu Y, Han X, Liu X. Effect of topical ropivacaine on the response to endotracheal tube during emergence from general anesthesia: A prospective randomized double-blind controlled study. BMC Anesthesiol. 2018;18:134.. doi: 10.1186/s12871-018-0601-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sale HK, Shendage VJ. Lignocaine and dexmedetomidine in attenuation of pressor response to laryngoscopy and intubation: A prospective study. Int J Sci Study. 2015;3:155–60.. [Google Scholar]
- 8.Kumar NRR, Jonnavithula N, Padhy S, Sanapala V, Naik VV. Evaluation of nebulised dexmedetomidine in blunting haemodynamic response to intubation. Indian J Anaesth. 2020;64:874–9. doi: 10.4103/ija.IJA_235_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanaty OM, El Metainy SA. A comparative evaluation of nebulized dexmedetomidine, nebulized ketamine, and their combination as premedication for outpatient pediatric dental surgery. Anesth Analg. 2015;121:167–71. doi: 10.1213/ANE.0000000000000728. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Yang F, Ye M, Liu H, Zhang J, Tian Q, et al. Intranasal dexmedetomidine is an effective sedative agent for electroencephalography in children. BMC Anesthesiol. 2020;20:61.. doi: 10.1186/s12871-020-00978-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saad BB, Tharwat AI, Ghobrial HN, Elfawal SM. Intranasal dexmedetomidine versus intranasal midazolam as pre-anesthetic medication in pediatric age group undergoing adenotonsillectomy. Ain-Shams J Anesthesiol. 2020;12:40.. [Google Scholar]
- 12.Panda S, Pujara J, Chauhan A, Varma A, venuthurupalli R, Pandya H, et al. Comparative study of intranasal dexmedetomidine v/s midazolam for sedation of pediatric patients during transthoracic echocardiography. Ann Card Anaesth. 2021;24:224–9. doi: 10.4103/aca.ACA_17_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain M, Arun N, Kumar S, Kumar A, Kumar R, Shekhar S. Effect of dexmedetomidine nebulization on attenuation of haemodynamic responses to laryngoscopy: Randomised controlled study. Indian J Anesth Analg. 2019;6:1235–40. [Google Scholar]
- 14.Thomas D, Chacko L, Raphael PO. Dexmedetomidine nebulisation attenuates post-operative sore throat in patients undergoing thyroidectomy: A randomised, double-blind, comparative study with nebulised ketamine. Indian J Anaesth. 2020;64:863–8. doi: 10.4103/ija.IJA_406_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adcock JJ, Douglas GJ, Garabette M, Gascoigne M, Beatch G, Walker M, et al. RSD931, a novel anti-tussive agent acting on airway sensory nerves. Br J Pharmacol. 2003;138:407–16. doi: 10.1038/sj.bjp.0705056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandazi AK, Louizos AA, Davilis DJ, Stivaktakis JM, Georgiou LG. Inhalational anesthetic technique in microlaryngeal surgery: A comparison between sevoflurane remifentanil and sevoflurane alfentanil anesthesia. Ann Otol Rhinol Laryngol. 2003;112:373–8. doi: 10.1177/000348940311200414. [DOI] [PubMed] [Google Scholar]
- 17.Jain S, Khan RM. Effect of peri-operative intravenous infusion of lignocaine on haemodynamic responses to intubation, extubation and post-operative analgesia. Indian J Anaesth. 2015;59:342–7. doi: 10.4103/0019-5049.158733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao W, Xi JH, Ju NY, Cui GX. Ropivacaine via trans-cricothyroid membrane injection inhibits the extubation response in patients undergoing surgery for maxillary and mandibular fractures. Genet Mol Res. 2014;13:1635–42. doi: 10.4238/2014.March.12.16. [DOI] [PubMed] [Google Scholar]
- 19.Stewart J, Kellett N, Castro D. The central nervous system and cardiovascular effects of levobupivacaine and ropivacaine in healthy volunteers. Anesth Analg. 2003;97:412–6. doi: 10.1213/01.ANE.0000069506.68137.F2. [DOI] [PubMed] [Google Scholar]
- 20.Shrivastava P, Kumar M, Verma S, Sharma R, Kumar R, Ranjan R, et al. Evaluation of nebulised dexmedetomidine given pre-operatively to attenuate hemodynamic response to laryngoscopy and endotracheal intubation: A randomised control trial. Cureus. 2022;14:e25223.. doi: 10.7759/cureus.25223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebastian B, Talikoti AT, Krishnamurthy D. Attenuation of haemodynamic responses to laryngoscopy and endotracheal intubation with intravenous dexmedetomidine: A comparison between two doses. Indian J Anaesth. 2017;61:48–54. doi: 10.4103/0019-5049.198404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selvaraj V, Manoharan KR. Prospective randomized study to compare between intravenous dexmedetomidine and esmolol for attenuation of hemodynamic response to endotracheal intubation. Anesth Essays Res. 2016;10:343–8. doi: 10.4103/0259-1162.181226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhiman T, Verma V, Kumar Verma R, Rana S, Singh J, Badhan I. Dexmedetomidine-ketamine or dexmedetomidine-midazolam nebulised drug combination as a premedicant in children: A randomised clinical trial. Turk J Anaesthesiol Reanim. 2022;50:380–7. doi: 10.5152/TJAR.2022.21298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anupriya J, Kurhekar P. Randomised comparison between the efficacy of two doses of nebulised dexmedetomidine for premedication in paediatric patients. Turk J Anaesthesiol Reanim. 2020;48:314–20. doi: 10.5152/TJAR.2019.78889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu W, Xu M, Lu H, Huang Q, Wu J. Nebulized dexmedetomidine-lidocaine inhalation as a premedication for flexible bronchoscopy: A randomized trial. J Thorac Dis. 2019;11:4663–70. doi: 10.21037/jtd.2019.10.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misra S, Behera BK, Mitra JK, Sahoo AK, Jena SS, Srinivasan A. Effect of preoperative dexmedetomidine nebulization on the hemodynamic response to laryngoscopy and intubation: A randomized control trial. Korean J Anesthesiol. 2021;74:150–7. doi: 10.4097/kja.20153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg. 1999;89:652–8. doi: 10.1097/00000539-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 28.Sant'Ambrogio G, Widdicombe J. Reflexes from airway rapidly adapting receptors. Respir Physiol. 2001;125:33–45. doi: 10.1016/s0034-5687(00)00203-6. [DOI] [PubMed] [Google Scholar]
- 29.Diachun CA, Tunink BP, BrockUtne JG. Suppression of cough during emergence from general anesthesia: Laryngotracheal lidocaine through a modified endotracheal tube. J Clin Anesth. 2001;13:447–51. doi: 10.1016/s0952-8180(01)00299-9. [DOI] [PubMed] [Google Scholar]
- 30.Niu J, Hu R, Yang N, He Y, Sun H, Ning R, et al. Effect of intratracheal dexmedetomidine combined with ropivacaine on postoperative sore throat: A prospective randomised double-blinded controlled trial. BMC Anesthesiol. 2022;22:144.. doi: 10.1186/s12871-022-01694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


