Figure 3.
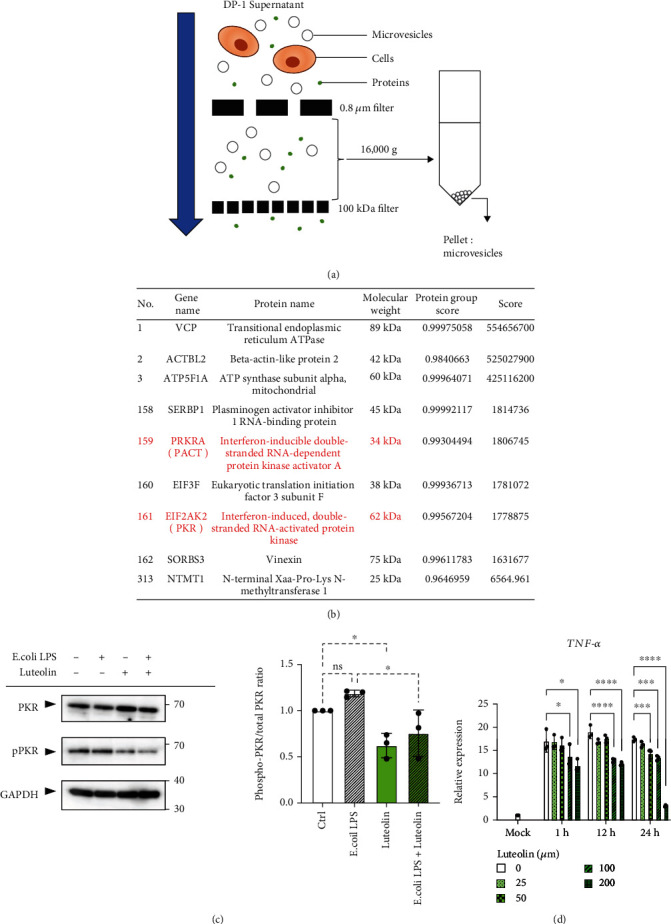
Identification of an endogenous PKR activator, a protein activator of interferon-induced PKR (PACT), using proteomic analysis and the inhibition of PKR phosphorylation by luteolin. (a) Schematic procedure for the isolation of microvesicles (MVs) from cell culture supernatant of dental pulp (DP-1) cells. (b) Representative stress granule component proteins in DP-1-derived MVs. Proteins from DP-1-derived MVs were identified using data-independent acquisition (DIA) proteomic analysis, subsequently followed by selection of stress granule component proteins using the Mammalian Stress Granules Proteome (MSGP) database (https://msgp.pt/). (c) Luteolin inhibits lipopolysaccharide- (LPS-) induced phosphorylation of protein kinase R (PKR) in DP-1 cells. DP-1 cells with or without luteolin (100 μM) pretreatment for 24 h were stimulated with E. coli LPS (100 ng/ml) for 3 h. GAPDH was used as the internal control (left). Relative phosphorylation of PKR was measured by quantifying the density of phospho-PKR to the densitometry of total PKR (n = 3) (right). (d) Luteolin inhibits DP-1 supernatant-induced expression of TNF-α mRNA in PMA-differentiated THP-1 (dTHP-1) cells. After pretreatment of DP-1 with the indicated concentration of luteolin for 24 h, dTHP-1 cells were stimulated with the supernatants derived from DP-1 for 3 h (n = 3). ∗p < 0.05, ∗∗∗p < 0.001. ∗∗∗∗p < 0.0001. Error bars represent means ± SD. Data were analyzed using independent unpaired two-tailed Student's t-tests.
