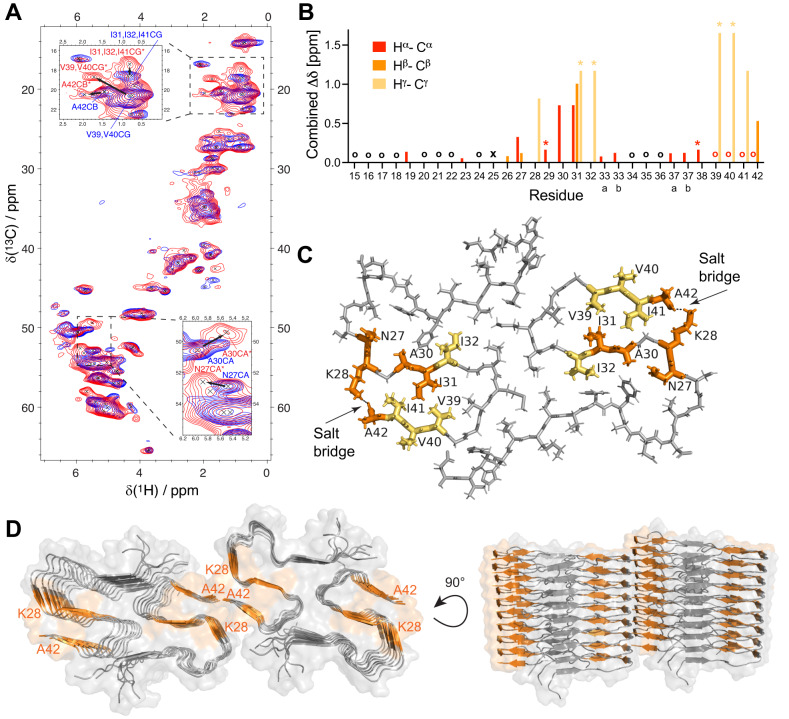
Fig. 3. Chemical shift changes of BRICHOS-Aβ42 fibrils and structural model.
A 1H,13C-correlation spectra of Aβ42 fibrils alone (blue) and BRICHOS-Aβ42 co-incubated fibrils (red). The doubled cross-peaks in the BRICHOS-Aβ42 spectrum are labeled in red with a star (*). The insets represent different zoomed regions. B Combined (1H and 13C) chemical shift changes between the new signals and those observable in the spectra of Aβ42 fibrils alone. Circles refer to overlap in the spectrum, stars to ambiguous assignments and crosses to residues with missing assignment. C Residues exhibiting signals with significant chemical shift doubling are colored in orange and yellow (for ambiguous assignments) on the fibril structure23, revealing that the last three β-strands, including the salt bridge between K28 and A42, are affected by the presence of BRICHOS. D Such residues are also illustrated onto the 3D model of tetrameric Aβ42 fibrils24.