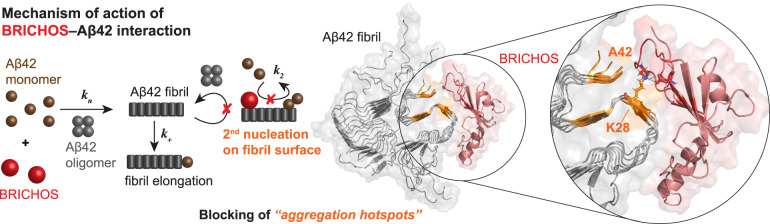
Fig. 5. Mechanistic model of BRICHOS binding to Aβ42 fibril and inhibition of secondary nucleation by blocking “aggregation hotspots” on the fibril surface.
The scheme shows a 3D model of a chemical-shift driven docking of BRICHOS (in red) onto the dimeric fibril structure23, where the N-terminal residues were added computationally. The simulated BRICHOS-Aβ42 complex reveals an ionic network interaction involving K28, which disturbs the salt bridge in the Aβ42 fibril between K28 and A42. Hence, the C-terminal β-strands and in particular the solvent exposed β-strand, stretching between residues 26–28, may represent the “aggregation hotspot” for secondary nucleation, which can be blocked by BRICHOS.