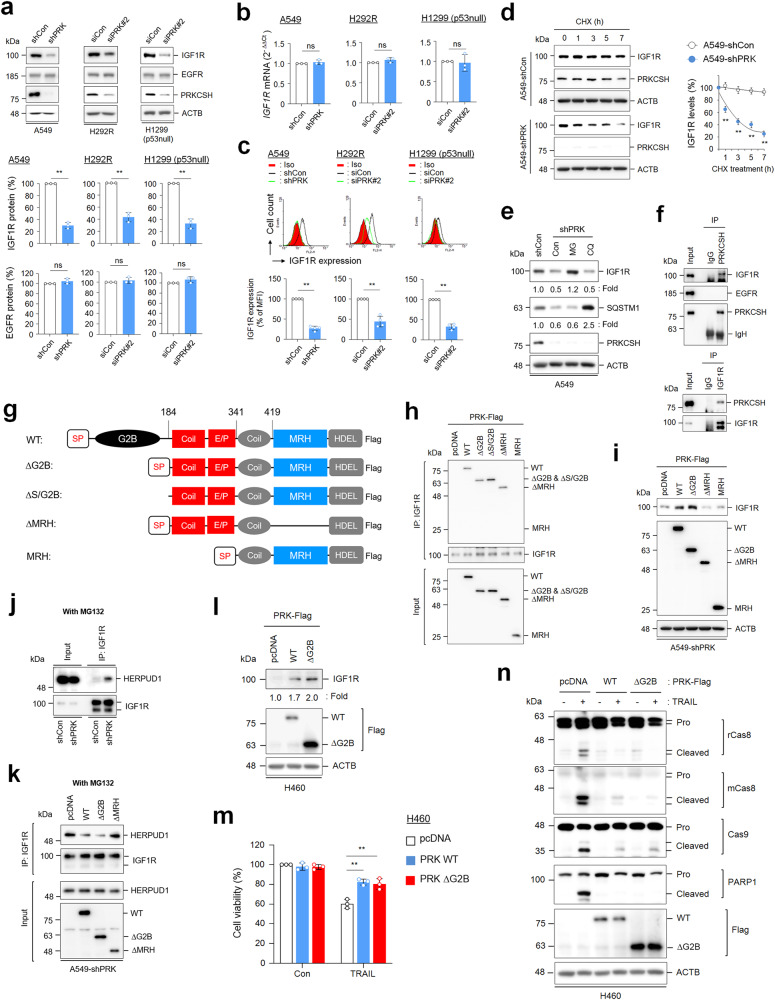
Fig. 7. The role of PRKCSH in the regulation of IGF1R protein half-life and its functional domain.
a Analysis of IGF1R and EGFR protein levels in PRKCSH-deficient cells. A549-shCon and A549-shPRK cells or other lung cancer cells, including H292R and H1299 (p53 null) transfected with siPRK#2 or siCon, were analyzed by immunoblotting using anti-IGF1R and EGFR antibodies. Representative immunoblots and quantitative analysis of IGF1R and EGFR protein levels in each cell line are shown. The quantitative data are shown as the means ± SDs of three independent assays. The statistical significance of differences between two groups was determined with the two-tailed Student’s t test. b Analysis of IGF1R mRNA expression levels in PRKCSH-deficient cells. The expression levels of IGF1R mRNA in A549-shCon and A549-shPRK cells or other lung cancer cells, including H292R and H1299 (p53 null) cells transfected with siPRK#2 or siCon, were analyzed by qPCR. Data are quantitative analysis of IGF1R mRNA expression in each cell line. c Analysis of IGF1R protein expression levels on the cell surface of PRKCSH-deficient cells. The expression of IGF1R protein on the cell surface of A549-shCon and A549-shPRK cells or other lung cancer cells, including H292R and H1299 (p53 null) cells transfected with siPRK#2 or siCon, was analyzed by flow cytometry. Representative flow cytometry data and quantitative analysis of IGF1R expression levels on the cell surface of each cell line are shown. d The effect of PRKCSH depletion on the IGF1R protein half-life in lung cancer cells. A549-shCon and A549-shPRK cells were treated with cycloheximide for the indicated times. The half-life of IGF1R protein in cell lysates was analyzed using immunoblotting. Representative immunoblots and quantitative analysis of IGF1R protein levels are shown. e The effect of proteasome or lysosome inhibitors on the regulation of IGF1R protein half-life in lung cancer cells. A549-shCon and A549-shPRK cells were treated with MG132 (proteasome inhibitor) or chloroquine (lysosome inhibitor) for 24 h. SQSTM1 was used as a lysosome inhibition control, and ACTB was used as a loading control. f Analysis of protein‒protein interactions among endogenous IGF1R, EGFR, and PRKCSH in lung cancer cells. The protein extracts from A549 cells were pulled down with anti-PRKCSH or anti-IGF1R antibodies, and the interacting proteins were identified by immunoblotting. g Scheme of the protein domains expressed in wild-type and mutant PRKCSH plasmids. WT, carboxyl-terminal Flag-tagged full-length PRKCSH; ΔG2B, deletion mutant of the G2B domain in WT; ΔS/G2B, deletion mutant of the signal peptide and G2B domain in WT; ΔMRH, deletion mutant of MRH in ΔG2B; MRH, deletion of the EP domain in ΔG2B. h Mapping domain of PRKCSH related to the interaction with IGF1R. A549 cells were transfected with WT PRKCSH and each PRKCSH deletion mutant plasmid. The protein extracts were pulled down with an anti-IGF1R antibody, and the interacting proteins were determined by immunoblotting with an anti-Flag antibody. i Mapping domain of PRKCSH associated with regulation of IGF1R protein half-life. A549-shPRK cells were transfected with WT and each deletion mutant of PRKCSH plasmids. IGF1R protein expression levels were determined by immunoblotting. j Analysis of the interaction between IGF1R and HERPUD1 in lung cancer cells. A549-shCon and A549-shPRK cells were treated with MG132 for 24 h. The protein extracts from each cell were pulled down with an anti-IGF1R antibody, and the interaction between IGF1R and HERPUDP1 protein was determined using immunoblotting. k The role of WT and each deletion mutant of PRKCSH in the inhibition of the interaction between IGF1R and HERPUD1. A549-shPRK cells were transfected with each plasmid, followed by treatment with MG132 for 24 h. The protein extracts from each cell were pulled down with an anti-IGF1R antibody, and the interaction between IGF1R and HERPUDP1 protein was determined using immunoblotting. l The effect of PRKCSH overexpression on IGF1R protein half-life. H460 lung cancer cells were transfected with WT or ΔG2B mutant plasmid. IGF1R protein expression levels were determined using immunoblotting. m-n The effect of PRKCSH overexpression on TNFSF-mediated tumor cell killing in H460 lung cancer cells. H460 cells were transfected with PRKCSH WT or ΔG2B mutant plasmid, followed by treatment with TRAIL for 24 h. TNFSF-mediated cytotoxicity was analyzed using MTT assay (m), and activation of caspases was analyzed using immunoblotting (n). **p < 0.01, *p < 0.05. NS, nonsignificant.