Abstract
Lymph node metastases are a major prognostic factor in survival of patients with oesophageal cancer. The number of lymph nodes removed during oesophagectomy has been previously proven to be associated with improved survival. The aim of this study was to examine the effect of lymph node harvest on survival specifically in pathologically node negative (pN0) patients with oesophageal cancer. Data were extracted from a prospectively populated single-surgeon database of oesophageal resections for cancer. All consecutive patients with pN0 were included. Patient-specific risk adjusted analysis of overall and disease-free survival was performed to identify the number of lymph nodes associated with improved survival. Inclusion criteria were met by 137 patients (49 squamous cell carcinoma and 88 adenocarcinoma). Adjusted for cancer stage, tumour (histological type, degree of differentiation, lympho-vascular invasion, neo-adjuvant therapy) and patient related factors (age, sex), increased lymph node number was associated with significant improvement in overall (P = 0.045) and disease free (P = 0.030) survival. Lymph node count ≥ 17 was associated with improved overall and disease-free survival. In this cohort of patients with pathologically node-negative oesophageal cancer, lymph node count of 17 or above was associated with significantly improved survival.
Subject terms: Oesophageal cancer, Surgical oncology
Introduction
Oesophageal cancer is the sixth leading cause of cancer related death worldwide with rates of adenocarcinoma on the rise1,2. Lymph node metastases are common even in early T1 disease and can be present in up to 40% of patients1. Extensive lymphadenectomy has been previously shown to improve survival in patients with oesophageal cancer1,3–5, however some studies have challenged this paradigm, especially in recipients of neo-adjuvant treatment3,6. Current National Comprehensive Cancer Network (NCCN) guidelines recommend minimum of 15 lymph node removal during oesophagectomy2. The rationale for adequate lymphadenectomy is that it may provide improved loco-regional disease control as well as allowing for adequate pathological disease staging.
Lymph node count was shown to predict improved survival in patients with gastric cancer who had no lymph node metastasis on final histopathology (pN0)7. This could be explained by the stage shift or removal of isolated tumour cell deposits not identified on pathologic examination, or combination effect. In lung cancer, node positivity, and therefore upstaging, was associated with increased number of lymph nodes examined by the pathologist8. Another study of patients with proximal urothelial cancers demonstrated improved survival in pN0 patients compared to pNx9.
The primary outcome of this study was to examine the effect of increased lymph node harvest in pN0 patients undergoing curative intent oesophagectomy for adenocarcinoma (AC) or squamous cell carcinoma (SCC) of the oesophagus on overall survival. Secondary outcomes included the effect of lymphadenectomy on disease specific survival and subgroup (AC and SCC) and stage specific survival.
Patients and methods
Data were extracted from a prospectively populated single surgeon database of oesophageal cancers between 1991 and 2016. During this period, a total of 578 patients underwent oesophagectomy for malignancy (excluding gastrointestinal stromal tumours) and this included 416 for AC and 162 for SCC. All consecutive patients who underwent oesophagectomy for AC or SCC and pathologically staged as N0 were included. Surgery was a standardised open Ivor-Lewis oesophagectomy with two field node dissection and adventitial mediastinal dissection.
Number of lymph nodes were reported by a specialist pathologist after examining all lymph nodes cut in a way to allow for maximum surface assessment and immunohistochemistry staining, which was routinely performed.
All patients were routinely followed up post-operatively with a clinical review by the operating surgeon at 6 weeks, 3 months, 6 months, then every 6 months for the first two years, then annually for up to 5 years post-operatively. Routine follow up imaging or endoscopic surveillance was not performed except upon strong patient preference or other clinical indication (for example pre-operative small lung nodule for surveillance). Patients who developed new symptoms or signs concerning for recurrence were promptly evaluated by imaging, endoscopy and biopsies (as was clinically appropriate). Following the 5 year period patients, were followed up on “as required” basis.
The database was approved by the Concord Hospital Human Research Ethics Committee (approval LNR/12/CRGH/248). All participants provided written informed consent to participate in research. Study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Overall survival (OS) was analysed using time to death or last follow-up. Disease-free survival (DFS) was measured using time from treatment to tumour recurrence confirmed on imaging or endoscopy, or confirmation of no evidence of disease (NED) or death of the patient.
Patient demographics included sex and age at diagnosis. Tumour characteristics included type (SCC or AC), location, histological grade, presence of lympho-vascular invasion (LVI) and American Joint Committee on Cancer (AJCC) 8th edition stage. The entire cohort was re-staged according to the AJCC 8th edition when it became available by one of the authors (CWK)10.
Statistical analyses were performed using SPSS 26 (IBM, New York). A two-sided P-value of < 0.05 was considered significant. Initial analysis was performed using < 15 nodes and ≥ 15 nodes as per NCCN guidelines, later analysis was performed to determine if higher number of nodes was associated with improved survival. Subgroup analysis was performed for each histological subtype (AC and SCC) and tumour stage. Using the Kaplan–Meier method and Log Rank test overall survival and disease-free survival between each lymph node group was compared. Potential confounders were adjusted using a Cox proportional hazards regression model. Furthermore, the Mann–Whitney U test was used to evaluate the treatment effect of neoadjuvant radiotherapy on number of resected lymph nodes during surgery.
Results
Table 1 summarises the demographics of the 137 patients included in the study. Most were male (68.6%) and the median age was 66 years (IQR 60–73). The median number of lymph nodes resected during surgery was 17 (IQR 12–25). Most tumours were adenocarcinoma (64%) and less than 10% of patients underwent neoadjuvant radiotherapy. The median follow-up time for the whole cohort was 8.1 years.
Table 1.
Characteristics of patients with pN0 oesophageal cancer (n = 137).
| Characteristic | Number of patients | Percentage |
|---|---|---|
| Age | ||
| < 65 years | 55 | 40.1 |
| ≥ 65 years | 82 | 59.9 |
| Sex | ||
| Female | 43 | 31.4 |
| Male | 94 | 68.6 |
| Histology | ||
| Adenocarcinoma | 88 | 64.2 |
| Squamous cell carcinoma | 49 | 35.8 |
| Tumour location | ||
| Upper oesophagus | 5 | 3.6 |
| Middle oesophagus | 28 | 20.4 |
| Lower oesophagus | 56 | 40.9 |
| Gastro-oesophageal junction | 48 | 35.0 |
| Histological grade | ||
| G1 (well differentiated) | 19 | 13.9 |
| G2 (moderately differentiated) | 74 | 54.0 |
| G3 (poorly differentiated) | 44 | 32.1 |
| Presence of lymphovascular invasion | ||
| No | 113 | 82.5 |
| Yes | 24 | 17.5 |
| Tumour stage (AJCC 8th edition) | ||
| I | 64 | 46.7 |
| II | 47 | 34.3 |
| III | 23 | 16.8 |
| IV | 3 | 2.2 |
| Number of nodes resected | ||
| < 17 | 66 | 48.2 |
| ≥ 17 | 71 | 51.8 |
| Neoadjuvant therapy | ||
| None | 69 | 50.3 |
| Radiotherapy | 13 | 9.5 |
| Chemotherapy | 43 | 31.4 |
| Chemoradiotherapy | 12 | 8.8 |
| Adjuvant chemotherapy | ||
| No | 121 | 88.3 |
| Yes | 16 | 11.7 |
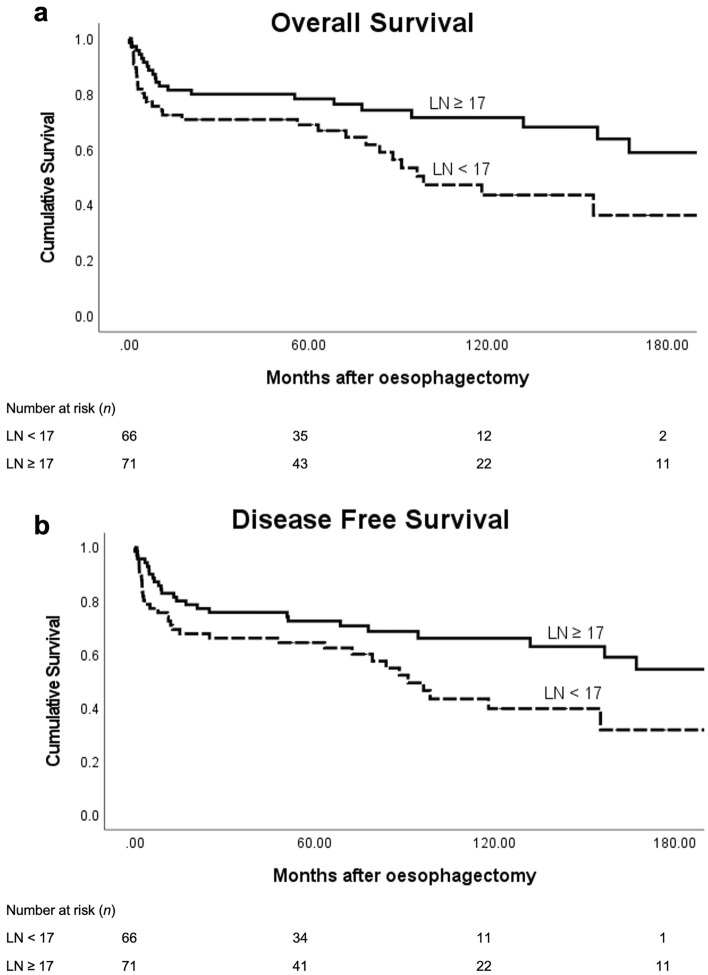
The median OS was 13.7 years with a 5-year actuarial survival rate of 72%. Kaplan–Meier analysis demonstrated a significant difference in OS (Log Rank, P = 0.045, Fig. 1a) and DFS (Log Rank, P = 0.030, Fig. 1b) between the lymph node groups (Fig. 1). The median OS and 5-year actuarial survival rates in patients with less than 17 and 17 or more nodes resected was 8.6 years and 67% and 17.3 years and 76% respectively.
Figure 1.
Kaplan–Meier survival curves based on number of resected lymph nodes (LN) in pN0 oesophageal cancer (n = 137; 17 or more LNs versus less than 17 LNs). (a) Overall survival (Log Rank, P = 0.045), (b) Disease Free survival (Log Rank, P = 0.030). Numbers below graphs indicate the number of patients at risk at each time point for the LN groups.
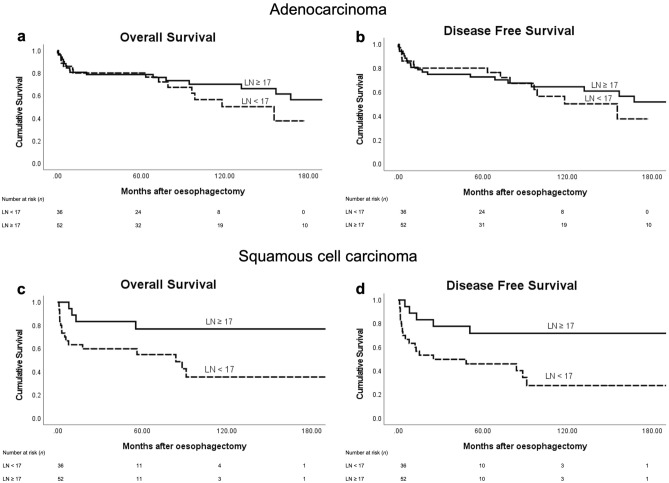
Subgroup analyses for OS and DFS for both histological subtypes were performed (Fig. 2). However, a difference for OS and DFS only reached statistical significance in the SCC group (AC: Log Rank, OS P = 0.322, DFS P = 0.622 vs SCC: Log Rank, OS P = 0.047, DFS P = 0.013).
Figure 2.
Kaplan–Meier survival curves for histological subtypes (adenocarcinoma, n = 88 vs squamous cell carcinoma, n = 49) based on number of resected lymph nodes (LN) in pN0 oesophageal cancer (17 or more LNs versus less than 17 LNs). (a) Adenocarcinoma—Overall survival (Log Rank, P = 0.322), (b) Adenocarcinoma—Disease Free survival (Log Rank, P = 0.622). (c) Squamous cell carcinoma—Overall survival (Log Rank, P = 0.047), (d) Squamous cell carcinoma—Disease Free survival (Log Rank, P = 0.013). Numbers below graphs indicate the number of patients at risk at each time point for the LN groups.
Subgroup analysis for tumour AJCC stage revealed a significant improvement in OS and DFS for stage II patients (n = 47) with 17 or more lymph nodes were removed (Log Rank, OS P = 0.029, DFS P = 0.032). Improvement in OS or DFS based on number of lymph nodes resected for AJCC stages I, III and IV did not reach statistical significance.
Univariate and multivariate hazard ratios (HRs) from Cox regression analysis are shown in Table 2. After adjusting for ten potential confounders (age, sex, tumour histology, location, histological grade, presence of lympho-vascular invasion, stage, neoadjuvant chemotherapy and radiotherapy and adjuvant chemotherapy), a greater number of resected lymph nodes was associated with improved overall survival. In particular, when compared to resecting less than 17 lymph nodes, resecting 17 or more nodes (HR 0.45, 95% CI 0.22–0.92) was associated with a reduced hazard of death. Furthermore, patients with stage IV tumours (3 patients) were independently associated with greater hazard of death (HR 5.98, 95% CI 1.07–33.65).
Table 2.
Univariate and multivariate predictors of overall survival in pN0 oesophageal cancer.
| Characteristic | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | ||||
| < 65 years | Reference | Reference | ||
| ≥ 65 years | 0.89 (0.52–1.51) | 0.659 | 0.75 (0.41–1.38) | 0.354 |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 0.72 (0.41–1.25) | 0.238 | 0.75 (0.35–1.59) | 0.447 |
| Histology | ||||
| Adenocarcinoma | Reference | Reference | ||
| Squamous cell carcinoma | 1.35 (0.77–2.36) | 0.294 | 0.31 (0.10–0.99) | 0.048* |
| Tumour location | ||||
| Upper oesophagus | Reference | Reference | ||
| Middle oesophagus | 2.21 (0.51–9.69) | 0.292 | 4.76 (0.92–24.61) | 0.063 |
| Lower oesophagus | 0.66 (0.15–2.94) | 0.588 | 0.95 (0.18–4.87) | 0.949 |
| Gastro-oesophageal junction | 1.06 (0.25–4.55) | 0.940 | 1.28 (0.25–6.65) | 0.772 |
| Histological grade | ||||
| G1 (well differentiated) | Reference | Reference | ||
| G2 (moderately differentiated) | 1.85 (0.71–4.85) | 0.211 | 1.66 (0.53–5.25) | 0.388 |
| G3 (poorly differentiated) | 2.35 (0.89–6.21) | 0.086 | 2.28 (0.76–6.80) | 0.141 |
| Presence of lymphovascular invasion | ||||
| No | Reference | Reference | ||
| Yes | 1.06 (0.53–2.12) | 0.872 | 1.32 (0.60–2.91) | 0.495 |
| Tumour stage (AJCC8) | ||||
| I | Reference | Reference | ||
| II | 1.63 (0.90–2.94) | 0.105 | 1.19 (0.59–2.39) | 0.635 |
| III | 0.74 (0.30–1.83) | 0.517 | 0.36 (0.12–1.10) | 0.073 |
| IV | 3.54 (0.82–15.26) | 0.091 | 5.98 (1.07–33.56) | 0.042* |
| Number of nodes resected | ||||
| < 17 | Reference | Reference | ||
| ≥ 17 | 0.58 (0.34–0.99) | 0.048* | 0.45 (0.22–0.92) | 0.028* |
| Neoadjuvant radiotherapy | ||||
| No | Reference | Reference | ||
| Yes | 2.40 (1.17–4.92) | 0.018* | 0.70 (0.21–2.32) | 0.564 |
| Neoadjuvant chemotherapy | ||||
| No | Reference | Reference | ||
| Yes | 1.61 (0.92–2.83) | 0.099 | 3.16 (1.24–8.03) | 0.016* |
| Adjuvant chemotherapy | ||||
| No | Reference | Reference | ||
| Yes | 0.66 (2.60–1.66) | 0.375 | 0.35 (0.11–1.12) | 0.076 |
HR hazard ratio, CI confidence interval.
*Significant P-value.
Patients who received neoadjuvant radiotherapy had a significantly lower number of lymph nodes harvested than those who did not (mean 8.7 vs 19.4, U = 292, P < 0.001). Although neoadjuvant radiotherapy was associated with a significantly higher unadjusted hazard of death (HR 2.40, 95% CI 1.17–4.92), after adjusting for potential confounders, it had no effect on overall survival (HR 0.70, 95% CI 0.21–2.32). Interestingly, patients who received neoadjuvant chemotherapy had a significantly higher adjusted hazard of death (HR 3.16, 95% CI 1.24–8.03). However, adjuvant chemotherapy was not an independent predictor of overall survival (HR 0.35, 95% CI 0.11–1.12).
Discussion
The results of this study highlight the importance of lymphadenectomy in either improving survival in the pathologically node-negative subgroup or upstaging true node positive patients with cancer of the oesophagus.
A key finding in this cohort was that a nodal harvest of 17 or greater was associated with improved survival.
Increased number of lymph nodes in the examined specimen has been shown to increase pathological stage8. Incomplete pathologic lymph node examination and under staging patients as a result can falsely worsen stage-per-stage survival. The number of nodes counted in the specimen may reflect the meticulousness of pathological examination and thus improve accuracy of pathological staging, potentially changing stage-based survival.
Whist this difference may be contributed to simply improving accuracy of staging, it is possible that lymphadenectomy improves survival via other pathways. For example, controlling potential micro-metastasis not detected on pathological examination or isolated tumour cell deposits (ITC) which are currently classified as pN011. Thorough lymphadenectomy may offer superior local disease control by removing microscopic deposits possibly not detected by the pathologist. Studies suggested that re-evaluation of lymph nodes with additional immunohistochemistry has been shown to detect occult lymph node disease in up to 17% of patients staged as pN012. Although the effect of ITC in oesophageal carcinoma prognosis remains a subject of debate, it seems more than likely that their prognostic value is similar to that of the micrometastasis13,14. It is therefore likely that, for some patients, extensive lymphadenectomy offers improvement in survival by removal of pathologically undetected metastasis or detected ITC (which are reported as pN0).
Neoadjuvant radiotherapy was associated with significantly reduced lymph node count in this study without effect on overall survival. Other studies showed complete pathological response to systemic treatment (in primary tumour and lymph node disease) improves survival, whereas partial or non-responders to neo-adjuvant treatment have significantly worse disease free and overall survival15,16. For patients with poor response to neo-adjuvant treatment radical surgery with extensive lymphadenectomy could be the only chance of cure.
Subgroup analysis based on tumour type (AC vs SCC) has shown a trend towards improvement in survival with increased lymph node harvest in both groups, however this difference was only significant in the SCC group. Similar results have been demonstrated in node negative head and neck SCC17. Extensive 3-field lymphadenectomy possibly improves survival in node-positive oesophageal SCC18. Perhaps lymphadenectomy is even more important in patients with SCC possibly offering better disease control to that of radiotherapy?
Survival benefit was most pronounced in patients with stage II disease. This significant difference in OS and DFS may support the hypothesis of increasing stage accuracy with increased nodal count most evident in patients with T2 and T3 tumours (stage II). It may be most noticeable in this group as the node status is the stronger determinant of survival in patients with larger tumours. Lymph node involvement can be an indicator of increased risk of distant metastasis which account for significant disease specific mortality in oesophageal cancer.
Management of regional lymph nodes appears to play a key role in improving outcomes of patients with oesophageal cancer19. Future research should be directed in risk stratifying patients based on molecular classifiers20. This may allow less invasive treatment in some patients with early tumours (T1) and those having complete clinical response to neoadjuvant treatment. Until the accuracy of non-invasive methods of assessing for complete clinical response improve, surgery with complete lymphadenectomy (upfront or salvage) remains standard treatment21,22. The concept of a sentinel lymph node has been proven in oesophageal cancer23. It is possible that in the future endoscopic mucosal resection and sentinel lymph node biopsy can become a standard of care in select complete responders and T1b patients to reduce the morbidity of oesophagectomy. Although the relatively high rate of skip nodal metastasis remains of concern. Data in this study suggested extended lymphadenectomy remains the gold standard for curability and lesser lymphadenectomy may risk reducing survival. This study supports current NCCN recommendation in minimum of 15 lymph node harvest for all patients undergoing oesophagectomy for cancer even if clinically staged as N0 and suggests that in pN0 subgroup removing even more nodes (≥ 17 in this group) can be beneficial2.
Active surveillance of SCC with clinical complete response and salvage surgery in recurrent disease has been proposed by some authors with promising results22. Further research is needed to assess whether survival benefit of removal of ≥ 15 nodes is sustained in patients undergoing salvage surgery where lymph node count is expected to be lower.
The strengths of this study include single surgeon database allowing for a standardised procedure and treatment protocols that reflect current management (supporting generalisability), and robust prospective data collection. The small number of patients receiving neoadjuvant treatment in this cohort limits bias of effects of systemic treatment by complete pathologic response. The main limitations of this study are retrospective cohort design, and the lack of data on circumferential resection margin.
Conclusion
In this cohort of patients with pN0 oesophageal cancer lymph node count of ≥ 17 was associated with improved overall survival and disease-free survival. Possible explanations for this include improved staging accuracy and improved local disease control and until this can be quantified, radical lymphadenectomy will remain the treatment of choice.
Author contributions
O.K. was the lead author, drafted majority of the manuscript, reviewed and analysed data. S.P. performed data analysis, literature research and manuscript editing. J.P. maintained database, participated in critical analysis and literature search. C.K. maintained database and re-staged entire database once AJCC 8 became available. G.L.F. is a senior author, created database and enrolled patients, reviewed and provided senior supervision to the creation of this manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Raw data can be made available on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang Y, et al. Prognostic value of the extent of lymphadenectomy for esophageal cancer-specific survival among T1 patients. BMC Cancer. 2021;21:403. doi: 10.1186/s12885-021-08080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajani JA, et al. Esophageal and esophagogastric junction cancers, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2019;17:855–883. doi: 10.6004/jnccn.2019.0033. [DOI] [PubMed] [Google Scholar]
- 3.Koen Talsma A, et al. Lymph node retrieval during esophagectomy with and without neoadjuvant chemoradiotherapy: Prognostic and therapeutic impact on survival. Ann. Surg. 2014;260:786–792. doi: 10.1097/SLA.0000000000000965. [DOI] [PubMed] [Google Scholar]
- 4.Peyre CG, et al. The number of lymph nodes removed predicts survival in esophageal cancer: An international study on the impact of extent of surgical resection. Ann. Surg. 2008;248:549–556. doi: 10.1097/SLA.0b013e318188c474. [DOI] [PubMed] [Google Scholar]
- 5.Kelty CJ, Kennedy CW, Falk GL. Ratio of metastatic lymph nodes to total number of nodes resected is prognostic for survival in esophageal carcinoma. J. Thorac. Oncol. 2010;5:1467–1471. doi: 10.1097/JTO.0b013e3181e8f6b1. [DOI] [PubMed] [Google Scholar]
- 6.van der Schaaf M, Johar A, Wijnhoven B, Lagergren P, Lagergren J. Extent of lymph node removal during esophageal cancer surgery and survival. J. Natl. Cancer Inst. 2015;107:43. doi: 10.1093/jnci/djv043. [DOI] [PubMed] [Google Scholar]
- 7.MingHua Z, et al. Impact of lymph nodes examined on survival in ypN0 gastric cancer patients: A population-based study. J. Gastrointest. Surg. 2021;25:919–925. doi: 10.1007/s11605-020-04579-6. [DOI] [PubMed] [Google Scholar]
- 8.Osarogiagbon RU, et al. Survival implications of variation in the thoroughness of pathologic lymph node examination in american college of surgeons oncology group Z0030 (Alliance) Ann. Thorac. Surg. 2016;102:363–369. doi: 10.1016/j.athoracsur.2016.03.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roscigno M, et al. Impact of lymph node dissection on cancer specific survival in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy. J. Urol. 2009;181:2482–2489. doi: 10.1016/j.juro.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Amin M. AJCC Cancer Staging Manual. 8. American College of Surgeons; 2017. [Google Scholar]
- 11.MacGuill MJ, Barrett C, Ravi N, MacDonald G, Reynolds JV. Isolated tumour cells in pathological node-negative lymph nodes adversely affect prognosis in cancer of the oesophagus or oesophagogastric junction. J. Clin. Pathol. 2007;60:1108–1111. doi: 10.1136/jcp.2006.044149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen SB, et al. Prognostic value of occult lymph node metastases in patients with completely resected esophageal squamous cell carcinoma. Sci. Rep. 2020;10:22007. doi: 10.1038/s41598-020-79073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lütken CD, Fiehn AK, Federspiel B, Achiam MP. Impact of isolated tumor cells in regional lymph nodes in adeno-and squamous cell carcinoma of the esophagus and the esophagogastric junction: A systematic review. Pathol. Res. Pract. 2019;215:849–854. doi: 10.1016/j.prp.2019.01.040. [DOI] [PubMed] [Google Scholar]
- 14.Thompson SK, Ruszkiewicz AR, Jamieson GG, Sullivan TR, Devitt PG. Isolated tumor cells in esophageal cancer: Implications for the surgeon and the pathologist. Ann. Surg. 2010;252:299–306. doi: 10.1097/SLA.0b013e3181e61e15. [DOI] [PubMed] [Google Scholar]
- 15.Blackham AU, et al. The prognostic value of residual nodal disease following neoadjuvant chemoradiation for esophageal cancer in patients with complete primary tumor response. J. Surg. Oncol. 2015;112:597–602. doi: 10.1002/jso.24050. [DOI] [PubMed] [Google Scholar]
- 16.Shridhar R, et al. Lymph node harvest in esophageal cancer after neoadjuvant chemoradiotherapy. Ann. Surg. Oncol. 2013;20:3038–3043. doi: 10.1245/s10434-013-2988-4. [DOI] [PubMed] [Google Scholar]
- 17.Tsai CJ, et al. Association of number of dissected lymph nodes with survival in clinically node-negative oral cavity squamous cell carcinoma patients undergoing primary surgery: A population-based analysis. JAMA Otolaryngol. Head Neck Surg. 2017;143:1049–1052. doi: 10.1001/jamaoto.2017.0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye T, Sun Y, Zhang Y, Zhang Y, Chen H. Three-field or two-field resection for thoracic esophageal cancer: A meta-analysis. Ann. Thorac. Surg. 2013;96:1933–1941. doi: 10.1016/j.athoracsur.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda S, Takeuchi M, Kawakubo H, Kitagawa Y. Lymph node metastatic patterns and the development of multidisciplinary treatment for esophageal cancer. Dis. Esophagus. 2023;36:4. doi: 10.1093/dote/doad006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blum Murphy M, et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: the association with baseline variables and survival: The University of Texas MD Anderson Cancer Center experience. Cancer. 2017;123:4106–4113. doi: 10.1002/cncr.30953. [DOI] [PubMed] [Google Scholar]
- 21.Eyck BM, et al. Accuracy of detecting residual disease after neoadjuvant chemoradiotherapy for esophageal cancer: A systematic review and meta-analysis. Ann. Surg. 2020;271:245–256. doi: 10.1097/SLA.0000000000003397. [DOI] [PubMed] [Google Scholar]
- 22.Castoro C, et al. Complete clinical response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic oesophagus: Is surgery always necessary? J. Gastrointest. Surg. 2013;17:1375–1381. doi: 10.1007/s11605-013-2269-3. [DOI] [PubMed] [Google Scholar]
- 23.Nagaraja V, Eslick GD, Cox MR. Sentinel lymph node in oesophageal cancer: A systematic review and meta-analysis. J. Gastrointest. Oncol. 2014;5:127–141. doi: 10.3978/j.issn.2078-6891.2014.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Raw data can be made available on request.