Summary
Organochlorides are a crucial class of electrophiles in organic synthesis. Here, we present a protocol for the cross-electrophile coupling of aryl chlorides with unactivated alkyl chlorides, facilitated by an iron/B2pin2 catalytic system. We describe steps for the coupling of aryl chlorides with alkyl chlorides, followed by purification of products. This protocol can produce alkylated products with up to 81% yield.
For complete details on the use and execution of this protocol, please refer to Zhang et al.1
Subject areas: Chemistry, Material sciences, Earth sciences
Graphical abstract

Highlights
-
•
Coupling reaction of aryl chlorides with unactivated alkyl chlorides under iron catalysis
-
•
B2pin2 promoted cross-electrophile coupling reaction
-
•
Compatible with diverse functional groups
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Organochlorides are a crucial class of electrophiles in organic synthesis. Here, we present a protocol for the cross-electrophile coupling of aryl chlorides with unactivated alkyl chlorides, facilitated by an iron/B2pin2 catalytic system. We describe steps for the coupling of aryl chlorides with alkyl chlorides, followed by purification of products. This protocol can produce alkylated products with up to 81% yield.
Before you begin
Organochlorides are a significant class of electrophiles due to their abundance, diverse structures, and ready accessibility. Despite these advantages, they are less frequently employed as coupling partners in cross-electrophile coupling reactions, mainly due to the high dissociation energy of the C-Cl bond. In recent years, efforts have been made to explore the use of organochlorides in such reactions. In 2018, Zhang and colleagues reported a nickel-catalyzed reaction involving aryl chlorides and HCF2Cl, showcasing high efficiency and a broad substrate scope.2 The Weix group, using an unconventional PyBCamCN ligand, achieved the cross-coupling of aryl chlorides with primary alkyl chlorides under nickel catalysis, in which only one example involving a secondary alkyl chloride was presented with low efficiency.3 Subsequently, MacMillan reported a similar study using photoredox/nickel catalysis. In this approach, a superior silane reagent induced the formation of alkyl radicals under mild photocatalytic conditions, thereby broadening the utilization of organochlorides.4
Iron catalysis has received increasing attention due to its abundance, non-toxicity, and cost-effectiveness.5,6,7 The functionalization of inert bonds under iron catalysis presents a significant challenge, often encountering difficulties in oxidative addition reactions with electrophiles when using iron-based complexes for cross-electrophilic reactions.8,9 However, our recent investigation has revealed that iron/B2pin2 catalytic systems can overcome this challenge, enabling the borylation and alkylation of the inert C-O bond.10,11,12,13,14 Notably, the active iron species in these catalytic systems may be in-situ generated. This finding leads us to anticipate that these resulting active iron species could play a crucial role in achieving the cross-electrophilic coupling of organochlorides. Therefore, inspired by these advances, there is significant merit in developing a general method for iron-catalyzed cross-electrophile coupling reaction of aryl chlorides with alkyl chlorides, especially for the secondary alkyl chlorides. The outlined protocol showcases the steps for the synthesis of alkylated compounds through an iron-catalyzed cross-electrophile coupling reaction between aryl chlorides and alkyl chlorides (Scheme 1). Additionally, this protocol is suitable for gram-scale synthesis and late-stage functionalization of biomolecules. For the same experiment performed in batch, please see Zhang et al. (2023).1
Scheme 1.
General scheme of iron-catalyzed cross-electrophile coupling reaction of organochlorides
Preparation of the reagents and equipment
A complete list of reagents and equipment can be found in the “key resources table” part.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| 4-Chloro-1,1′-biphenyl (aryl chloride) | Energy Chemical | Cat#D051465 |
| 1-Chloro-4-methoxybutane (alkyl chlorine) | Bidepharm | Cat#BD57841 |
| FeBr2 | Meryer | Cat#M58644 |
| MeOLi | Adamas | Cat#87055A |
| Dry methyl tert-butyl ether (MTBE) | Adamas | Cat#28130O |
| Tetramethylethylenediamine (TMEDA) | J&K Scientific | Cat#255989 |
| Sodium iodide (NaI) | Bidepharm | Cat#BD40879 |
| B2pin2 | Adamas | Cat#79736B |
| 3-Chloropyridine (hetero aryl chloride) | Bidepharm | Cat#BD34390 |
| Chlorocyclopentane (alkyl chlorine) | Adamas | Cat#90496A |
| CataCXium AHI | Bidepharm | Cat#BD234433 |
| FeCl2 | Bidepharm | Cat#BD130332 |
| Other | ||
| Silica gel for chromatography, 200–300 mm | Bidepharm | Cat#BD01115913 |
| Chromatography column (Ø 17 mm, 305 mm) | Synthware Glass | Cat#C189173C |
| 5 mL syringe | J&K Scientific | Cat#972645 |
| Glass vial | Synthware Glass | Cat#F580825D |
| Electronic balance | Mettler Toledo | Cat#ME204 |
| Heating module | Boost | Cat#DZ-AHB-PRL |
| Magnetic stirrer | IKA | Cat#RTC basic |
| Thin-layer chromatography (TLC-plates 0.25 mm) | Nuo Tai | Cat#GF 254 |
| Rotary evaporator | IKA | Cat#RV 10 basic |
| Vacuum pump | Yong Hao | Cat#2XZ-4 |
| 400 MHz NMR spectrometer | Agilent | Cat#400-MR DD2 |
| Glove box | DELLIX | Cat#GP25S |
Step-by-step method details
Note: Protocol steps in this section have been partially modified from our previous work, Bai et al., (2022).15
Part 1: Procedure for the cross-electrophile coupling of (hetero)aryl chlorides with unactivated alkyl chlorides
Timing: 15 h
Timing: 20 h (for step 2)
In this step, the setup of the coupling reaction using (hetero)aryl chlorides as substrates has been accomplished. The full scope of the transformation is described in Zhang et al. (2023).1
-
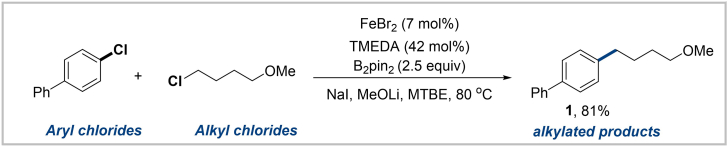
1.The coupling reaction of aryl chlorides with alkyl chlorides (Table 1; Scheme 2).
-
a.In one 8 mL of glass vial equipped with a magnetic stir bar.
-
b.Add FeBr2 (3.1 mg, 0.014 mmol, 7 mol %), B2pin2 (126.9 mg, 0.5 mmol, 2.5 equiv), LiOMe (41.8 mg, 1.1 mmol, 5.5 equiv), NaI (40.5 mg, 0.27 mmol, 1.35 equiv), and alkyl chloride (42.9 mg, 0.35 mmol, 1.75 equiv), aryl chlorides (37.7 mg, 0.2 mmol, 1.0 equiv), TMEDA (9.7 mg, 0.084 mmol, 42 mol %), freshly distilled MTBE (0.3 mL) in the glove box.
-
c.Seal the glass vial with a cap containing a polytetrafluoroethylene (PTFE)-lined silicone septum and move it out from the glove box.
-
d.Heat the reaction mixture in a heating module at 80°C for 15 h.
-
a.
CRITICAL: Seal the top of the glass vial with parafilm.
Note: LiOMe proves irreplaceable by alternative bases like t-BuOLi, t-BuONa, t-BuOK, MeONa, and MeOK in this reaction. DFT calculations suggested a mechanistic cycle involving a dilithium-iron complex. Furthermore, the synergistic interaction between iron and lithium may enhance the generation of alkyl radicals facilitated by the formation of a Li-Cl interaction. A Li cation-assisted single electron transfer (CASET) mechanism is likely involved in the catalytic cycle.
-
2.The coupling reaction of heteroaryl chlorides with alkyl chlorides (Table 2; Scheme 3).
-
a.In one 8 mL of glass vial equipped with a magnetic stir bar.
-
b.Add FeCl2 (2.5 mg, 0.02 mmol, 10 mol %), cataCXium AHI (9.7 mg, 0.02 mmol, 10 mol %), B2pin2 (126.9 mg, 0.5 mmol, 2.5 equiv), LiOMe (41.8 mg, 1.1 mmol, 5.5 equiv), alkyl chlorides (20.9 mg, 0.2 mmol, 1.0 equiv), chloropyridines (34.1 mg, 0.3 mmol, 1.5 equiv), freshly distilled MTBE (1.5 mL) in the glove box.
-
c.Seal the glass vial with a cap containing a PTFE-lined silicone septum and move it out from the glove box.
-
d.Heat the reaction mixture in a heating module at 80°C for 20 h.
-
a.
CRITICAL: Seal the top of the glass vial with parafilm.
Table 1.
Quantification of reagents, solvent, and product
| Reagent | Mw (g/mol) | m (mg) | n (mmol) | Equiv. | V (mL) | Conc (M) | Yield (%) |
|---|---|---|---|---|---|---|---|
| FeBr2 | 215 | 3.1 | 0.014 | 0.07 | |||
| B2pin2 | 253 | 126.9 | 0.5 | 2.5 | |||
| LiOMe | 37 | 41.8 | 1.1 | 5.5 | |||
| NaI | 149 | 40.5 | 0.27 | 1.35 | |||
| 1-Chloro-4-methoxybutane | 122 | 42.9 | 0.35 | 1.75 | |||
| 4-Chloro-1,1′-biphenyl | 188 | 37.7 | 0.2 | 1 | 0.66 | ||
| TMEDA | 116 | 9.7 | 0.084 | 0.42 | |||
| Dry MTBE | 0.3 | ||||||
| 4-(4-Methoxybutyl)-1,1′-biphenyl (1) | 240 | 38 | 0.16 | 81 |
1H NMR (400 MHz, CDCl3) δ 7.58 (d, J = 6.8 Hz, 2 H), 7.51 (d, J = 8.0 Hz, 2 H), 7.43 (t, J = 7.6 Hz, 2 H), 7.33 (t, J = 7.2 Hz, 1 H), 7.26 (d, J = 7.6 Hz, 2 H), 3.41 (t, J = 6.4 Hz, 2 H), 3.34 (s, 3 H), 2.68 (t, J = 7.2 Hz, 2 H), 1.84–1.55 (m, 4 H).
13C NMR (100 MHz, CDCl3) δ 141.7, 141.2, 138.8, 129.0, 128.8, 127.2, 127.1, 72.8, 58.8, 35.5, 29.5, 28.1.
Scheme 2.
Cross-electrophile coupling reaction of 4-chloro-1,1′-biphenyl with 1-chloro-4-methoxybutane
Table 2.
Quantification of reagents, solvent, and product
| Reagent | Mw (g/mol) | m (mg) | n (mmol) | Equiv. | V (mL) | Conc (M) | Yield (%) |
|---|---|---|---|---|---|---|---|
| FeCl2 | 126 | 2.5 | 0.02 | 0.1 | |||
| cataCXium AHI | 486 | 9.7 | 0.02 | 0.1 | |||
| B2pin2 | 253 | 126.9 | 0.5 | 2.5 | |||
| LiOMe | 37 | 41.8 | 1.1 | 5.5 | |||
| 3-Chloropyridine | 113 | 34.1 | 0.3 | 1.5 | |||
| Chlorocyclopentane | 104 | 20.9 | 0.2 | 1 | 0.14 | ||
| Dry MTBE | 1.5 | ||||||
| 3-Cyclopentylpyridine (2) | 147 | 23 | 0.16 | 80 |
1H NMR (400 MHz, CDCl3) δ 8.49 (s, 1 H), 8.41 (d, J = 4.8 Hz, 1 H), 7.52 (d, J = 7.6 Hz, 1 H), 7.19 (dd, J = 8.0, 4.8 Hz, 1 H), 3.04–2.93 (m, 1 H), 2.20–2.00 (m, 2 H), 1.91–1.77 (m, 2 H), 1.76–1.65 (m, 2 H), 1.64–1.47 (m, 2 H).
13C NMR (100 MHz, CDCl3) δ 149.2, 147.3, 141.7, 134.4, 123.4, 43.4, 34.5, 25.6.
Scheme 3.
Cross-electrophile coupling reaction of 3-chloropyridine with chlorocyclopentane
Part 2: Purification of the crude products
Timing: 2 h
In this step, the purification of desired products using (hetero)aryl chlorides as substrates has been accomplished.
-
3.After completing the reaction, cool the mixture to 25°C, then quench it with saturated aqueous ammonium chloride (sat. aq. NH4Cl, 1 mL).
-
a.Dilute the resulting mixture with 2 mL ethyl acetate.
-
b.Wash it with 2 mL saturated brine, and vigorously shake the separatory funnel.
-
c.Release pressure and allow the aqueous and organic phases to fully separate.
-
a.
-
4.
Transfer the separated organic and aqueous phases into two 20 mL flasks.
-
5.
Pour the aqueous phase back into the separatory funnel and add 2 mL ethyl acetate for extraction again.
-
6.Shake the separatory funnel vigorously, and allow the aqueous and organic phases to fully separate.
-
a.Transfer each organic and aqueous phases into their corresponding flasks.
-
a.
-
7.
Repeat steps 3 and 4 two times.
-
8.Transfer the organic layer to an Erlenmeyer flask and add Na2SO4.
-
a.Slowly shake the flask and filter the solution in a 25 mL round bottom flask.
-
a.
-
9.
Remove the solvent by rotatory evaporation (45°C, 152 mmHg, ∼15 min) to obtain the dried crude product.
-
10.Dissolve the dried crude product in 2 mL of dichloromethane and add 50 mg of silica into it.
-
a.Carefully swirl the flask and remove the solvent under vacuum (45°C, 235 mmHg, ∼15 min).
-
a.
-
11.
Purify the crude product by flash column chromatography (8 cm of silica, Ø of the column = 17 mm) using a 50:1 (by volume) mixture of PE/EA (∼100 mL).
-
12.Monitor the fractions by thin layer chromatography (TLC).
-
a.Collect the combined fractions containing the pure product.
-
b.Concentrate them under vacuum to deliver the desired alkylated compounds.
-
a.
Note: These coupling products are stable for half a year at 25°C–30°C.
Expected outcomes
A colorless oil, 4-(4-methoxybutyl)-1,1′-biphenyl 1 was obtained in 81% yield, totaling 39 mg.
A colorless oil, 3-cyclopentylpyridine 2 was obtained in 80% yield, totaling 24 mg.
Quantification and statistical analysis
Analytical data
4-(4-Methoxybutyl)-1,1'-biphenyl 1.1H NMR (400 MHz, CDCl3) δ 7.58 (d, J = 6.8 Hz, 2 H), 7.51 (d, J = 8.0 Hz, 2 H), 7.43 (t, J = 7.6 Hz, 2 H), 7.33 (t, J = 7.2 Hz, 1 H), 7.26 (d, J = 7.6 Hz, 2 H), 3.41 (t, J = 6.4 Hz, 2 H), 3.34 (s, 3 H), 2.68 (t, J = 7.2 Hz, 2 H), 1.84-1.55 (m, 4 H). 13C NMR (100 MHz, CDCl3) δ 141.7, 141.2, 138.8, 129.0, 128.8, 127.2, 127.1, 72.8, 58.8, 35.5, 29.5, 28.1.3-Cyclopentylpyridine 2.1H NMR (400 MHz, CDCl3) δ 8.49 (s, 1 H), 8.41 (d, J = 4.8 Hz, 1 H), 7.52 (d, J = 7.6 Hz, 1 H), 7.19 (dd, J = 8.0, 4.8 Hz, 1 H), 3.04-2.93 (m, 1 H), 2.20-2.00 (m, 2 H), 1.91-1.77 (m, 2 H), 1.76-1.65 (m, 2 H), 1.64-1.47 (m, 2 H).13C NMR (100 MHz, CDCl3) δ 149.2, 147.3, 141.7, 134.4, 123.4, 43.4, 34.5, 25.6.
Limitations
The protocol exhibits good efficiency with primary and secondary alkyl chlorides, while tertiary alkyl chlorides are not suitable for this protocol.
Troubleshooting
Problem 1
Step 1b & 2b: Can the yield of the reaction be improved by increasing the amount of catalyst?
Potential solution
Increasing the amount of catalyst has a negative impact on the efficiency of this reaction, resulting in an increased production of by-products from borylation and hydrogenation of organochlorides. Occasionally, for some substrates, reducing the catalyst loading will give the comparable results.
Problem 2
Step 1b & 2b: What are the possible reasons for the poor reproducibility of this reaction?
Potential solution
This reaction is slightly sensitive to both air and water. It is crucial to use freshly distilled MTBE as the solvent. Additionally, prolonged storage of MeOLi may result in a diminished yield.
Problem 3
Step 1b & 2b: After the reaction, the system will become very viscous or even dry. How to do workup of this reaction?
Potential solution
After quenching this reaction with a saturated ammonium chloride solution, add ethyl acetate to dilute the mixture, and stir quickly with a magnetic stirrer to facilitate layer separation.
Problem 4
Step 1b & 2b: How to store the catalysts and additives?
Potential solution
Catalysts and additives should be stored in the glove box.
Problem 5
Step 12a: What are the potential difficulties in the purification of the desired products?
Potential solution
Borylation side reaction always occurs during this transformation, and the corresponding by-products are difficult to separate from the desired alkylated products. These crude products could be purified by flash column chromatography, and then separated by thin-layer chromatography to get the high-purity products. Typically, borylated by-products exhibit higher polarity than the desired alkylation products, causing them to appear at the bottom of the TLC plate and facilitating easy separation.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Zhang Feng (fengzh@cqu.edu.cn).
Technical contact
Further information and requests for procedure should be directed to and will be fulfilled by the technical contact, Pengjie Xian (xianpengjie@126.com).
Materials availability
All other data supporting the finding of this study are available within the article, or from the lead contact upon reasonable request.
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
We are thankful for the financial support from the Affiliated Hospital of North Sichuan Medical College (no. 2022JB001), Research and Development Program Projects of North Sichuan Medical College (CBY23-QDA31), the National Natural Science Foundation of China (no. 22271031), and Medical Imaging Key Laboratory of Sichuan Province (nos. MIKL202201 and MIKL202202).
Author contributions
P.X. and Z.F. designed and wrote the protocol with inputs from all the authors. H.H., R.Z., and S.G. performed the experimental data.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Pengjie Xian, Email: xianpengjie@126.com.
Zhang Feng, Email: fengzh@cqu.edu.cn.
References
- 1.Zhang Y., Du P., Ji Y., Wang S., Zhu Y., Liu Z., He Y., Peng Q., Feng Z. Catalytic Cross-Electrophile Coupling of Aryl Chlorides with Unactivated Alkyl Chlorides: The Synergy of Iron and Li. Chem. 2023;9:3623–3636. [Google Scholar]
- 2.Xu C., Guo W.-H., He X., Guo Y.-L., Zhang X.-Y., Zhang X. Difluoromethylation of (Hetero) Aryl Chlorides with Chlorodifluoromethane Catalyzed by Nickel. Nat. Commun. 2018;9:1170. doi: 10.1038/s41467-018-03532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim S., Goldfogel M.-J., Gilbert M.-M., Weix D.-J. Nickel-Catalyzed Cross-Electrophile Coupling of Aryl Chlorides with Primary Alkyl Chlorides. J. Am. Chem. Soc. 2020;142:9902–9907. doi: 10.1021/jacs.0c02673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakai H.-A., Liu W., Le C.-C., MacMillan D.-W.-C. Cross-Electrophile Coupling of Unactivated Alkyl Chlorides. J. Am. Chem. Soc. 2020;142:11691–11697. doi: 10.1021/jacs.0c04812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L.-J., He Y., Yang Y., Guo J., Lu Z., Wang C., Zhu S., Zhu S.-F. Recent Advances in Mn, Fe, Co, and Ni-Catalyzed Organic Reactions. CCS Chem. 2023:1–48. doi: 10.31635/ccschem.023.202303412. [DOI] [Google Scholar]
- 6.Bauer I., Knölker H.J. Iron Catalysis in Organic Synthesis. Chem. Rev. 2015;115:3170–3387. doi: 10.1021/cr500425u. [DOI] [PubMed] [Google Scholar]
- 7.Chen J., Guo J., Lu Z. Recent Advances in Hydrometallation of Alkenes and Alkynes via the First Row Transition Metal Catalysis. Chin. J. Chem. 2018;36:1075–1109. [Google Scholar]
- 8.Fürstner A., Martin R., Krause H., Seidel G., Goddard R., Lehmann C.-W. Preparation, Structure, and Reactivity of Non-stabilized Organoiron Compounds. Implications for Iron-Catalyzed Cross Coupling Reactions. J. Am. Chem. Soc. 2008;130:8773–8787. doi: 10.1021/ja801466t. [DOI] [PubMed] [Google Scholar]
- 9.Sears J.-D., Neate P.-G.-N., Neidig M.-L. Intermediates and Mechanism in Iron-Catalyzed Cross-Coupling. J. Am. Chem. Soc. 2018;140:11872–11883. doi: 10.1021/jacs.8b06893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen Q., Chen S., Shi C., Chen S., Ji Y., Guo J., Liu Z., He Y., Feng Z. Iron-catalyzed decarbonylative borylation enables the one-pot diversification of (Hetero) Aryl and alkyl carboxylic acids. Cell Rep. Phys. Sci. 2022;3 doi: 10.1016/j.xpro.2022.101909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S., Wang Z., Geng S., Zhu H., Liu Z., He Y., Peng Q., Feng Z. Iron-catalyzed cross-electrophile coupling of inert C–O bonds with alkyl bromides. CCS Chem. 2023;5:1674–1685. [Google Scholar]
- 12.Geng S., Shi C., Guo B., Hou H., Liu Z., Feng Z. Recent Progress in Transition-Metal-Catalyzed Reductive Cross-Coupling Reactions Using Diboron Reagents as Reductants. ACS Catal. 2023;13:15469–15480. [Google Scholar]
- 13.Zhu Y., Chen S., Zhou Z., He Y., Liu Z., Liu Y., Feng Z. Iron/B2pin2 catalytic system enables the generation of alkyl radicals from inert alkyl C-O bonds for amine synthesis. Chinese. Chem. Lett. 2024;35 [Google Scholar]
- 14.Wang S., Sun M., Zhang H., Zhang J., He Y., Feng Z. Iron-catalyzed cross-electrophile coupling of inert C–O bonds with alkyl bromides. CCS Chem. 2021;3:2164–2173. [Google Scholar]
- 15.Bai M., Geng S., Chen S., Liu Z., Feng Z. A one-pot protocol for iron-catalyzed decarbonylative borylation of aryl and alkyl carboxylic acids. STAR Protoc. 2022;3 doi: 10.1016/j.xpro.2022.101909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.