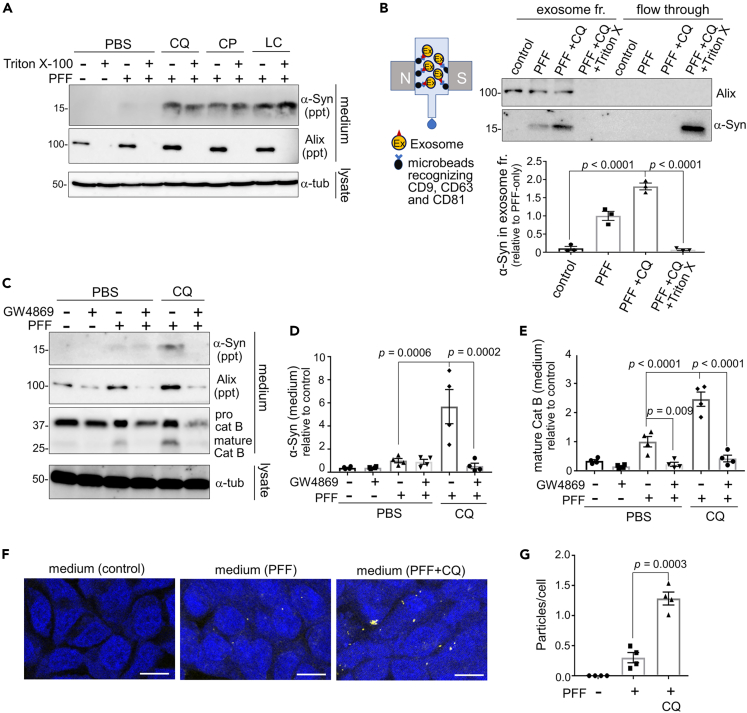
Figure 3.
Exosomes are involved in the release of insoluble and seed-competent α-synuclein
(A) Confirmation of insolubility of the α-synuclein released from RAW264.7 cells loaded with α-synuclein PFFs and exposed to the indicated lysosomotropic agents. The collected media were treated with Triton X-100 to solubilize exosomal structures and then ultracentrifuged. α-Synuclein, but not an exosomal marker Alix, remained detectable in the precipitate fraction after Triton X-100 treatment.
(B) Left: schematic diagram of ultracentrifugation-free exosome purification method using magnetic beads. Upper right: detection of α-synuclein and Alix in exosomal and flow-through fractions of media from CQ-treated RAW264.7 cells. Triton X-100 treatment prior to exosome purification resulted in the shift of α-synuclein from the exosome to flow-through fractions. Lower right: quantification of α-synuclein in the exosome fraction. Mean ± SEM, n = 3, one-way ANOVA with Tukey’s test.
(C) Inhibitory effect of an exosome inhibitor GW4869 on the release of insoluble α-synuclein, Alix and cathepsin B from RAW264.7 cells upon CQ treatment.
(D, E) Quantification of the release of insoluble α-synuclein and mature cathepsin B, as shown in C. Mean ± SEM, n = 4, one-way ANOVA with Tukey’s test.
(F) FRET fluorescence of α-synuclein biosensor cells treated with the media from RAW264.7 cells that had been treated with PFF or PFF+CQ. Bar = 10 μm.
(G) Quantification of the fluorescent dots in biosensor cells treated with the indicated media, as shown in F. Mean ± SEM, n = 4, one-way ANOVA with Tukey’s test. See also Figure S3.