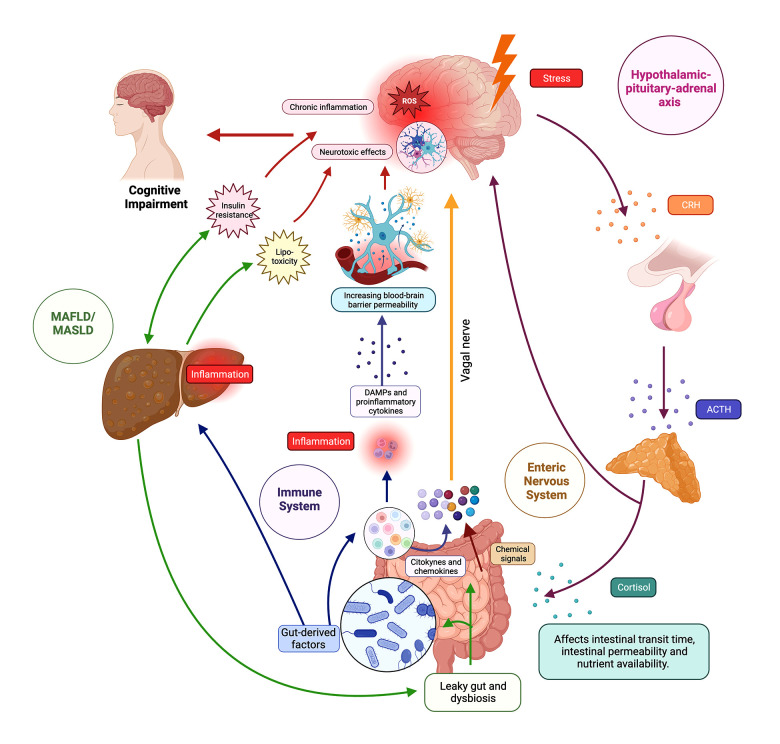
Figure 1. The brain–gut–liver axis.
The figure illustrates the intricate network of the brain–gut–liver axis, showing bidirectional communication pathways between the central nervous system (CNS), gastrointestinal tract, and liver. The axis involves neurological, endocrine, and immune components, influencing physiological and cognitive processes. In the neurological pathways, the enteric nervous system (ENS) senses the intestinal microenvironment, transmitting signals via vagal sensory nerves to the afferent neurons that express receptors for gut peptides (eg, CCK, ghrelin, leptin), influencing reactions and gut microbiota modulates gut peptide levels. In endocrine pathways, the hypothalamic–pituitary–adrenal (HPA) axis responds to stress, releasing corticotropin-releasing hormone (CRH) and stimulating cortisol production; cortisol impacts physiological processes, gut microbiota, and binds to glucocorticoid receptors (GR) in the brain. In immune pathways, gut-associated lymphoid tissue (GALT) communicates with the CNS through signaling molecules, influencing neuroimmune responses. Microbiome-derived products modulate immune cells and inflammation locally and systemically. Regarding the liver and cognitive impairment, the liver is connected to the gut via the portal vein and receives signals affecting liver health and the gut–brain axis. Disruptions in the axis, as seen in metabolic-associated fatty liver disease (MAFLD) and metabolic-associated steatosis of liver disease (MASLD), lead to “leaky gut”, which contributes to systemic inflammation, impacting cognitive function through interconnected pathways such as insulin resistance (IR) and lipotoxicity. ACTH – adrenocorticotropic hormone; CRH – corticotropin-releasing hormone, DAMPS – damage-associated molecular patterns; MAFLD – metabolic-associated fatty liver disease; MASLD – metabolic-associated steatotic liver disease. Created using Biorender.