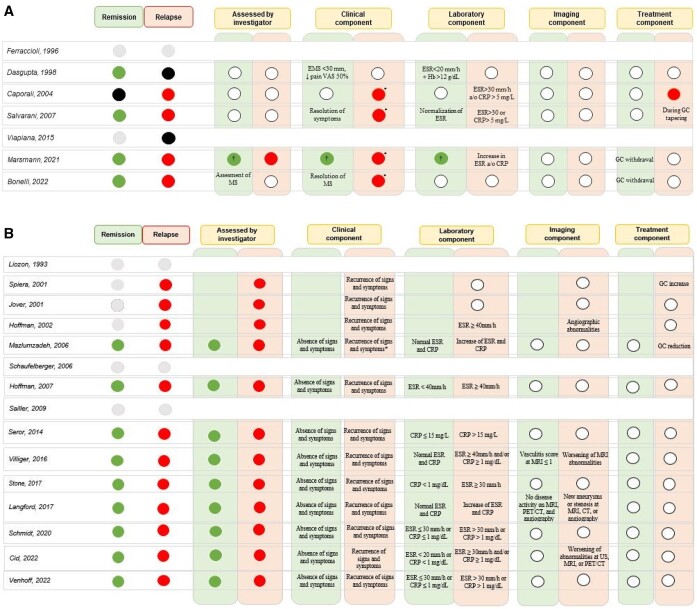
Figure 2.
Components used in defining remission and relapse in (A) PMR RCTs# and (B) GCA RCTs#. Grey circle: Remission and/or relapse used as outcomes but not defined in the study methods; Green circle: remission (or specific components) defined in the study methods; Red circle: relapse (or specific components) defined in the study methods; White circle: component not part of the definition of remission/relapse. Black circle: remission/relapse not used as an outcome. CRP, C-reactive protein; CT, computerized tomography; EMS, early morning stiffness; ESR, erythrocyte sedimentation rate; GC, glucocorticoids; Hb, haemoglobin; MS, morning stiffness; MRI, magnetic resonance imaging; PET, positron emission tomography; US, ultrasound; VAS, visual analogue scale. *, signs and symptoms of active polymyalgia rheumatica; †, remission defined by PMR-AS (PMR Activity Score) <10; a/o, and/or. #Only RCTs considering remission and/or relapse as an outcome are listed. Overall, in (A) both remission and relapse were mainly defined as a combination of clinical and laboratory parameters. None of the studies defined sustained remission. In (B) relapse was defined as the return of signs and symptoms and/or an increase of ESR/CRP after reduction of prednisone dosage followed by an improvement of signs and symptoms when GC dosage was increased. Recurrence was defined as the reappearance of GCA signs and symptoms and/or increase of the inflammatory markers in a GCA patients not receiving GC therapy for at least 1 month. Overall, in GCA RCTs, both remission and relapse were mainly defined as a combination of clinical and laboratory parameters