Table 3.
(continued)
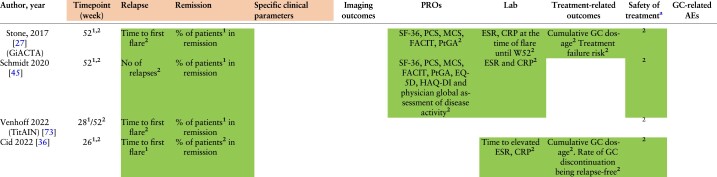
|
Overall, clinical and laboratory components were mainly used as outcomes and treatment targets. Assessment of clinical items was part of the study outcomes in 94.4% of the included RCTs, in terms of the evaluation of either remission, relapse or resolution of specific signs and symptoms.
Primary and secondary endpoints are expressed with numbers ‘1’ and ‘2’ inserted as exponential values in each outcome.
α2-g, alfa-2 globulins; ACTH, adrenocorticotropic hormone; AEs, adverse events; BMC, bone mineral content; BMD, bone mineral density; CRP, C-reactive protein; DEXA, dual-energy X-ray absorption; EQ-5D, EuroQol-5D; ESR, erythrocyte sedimentation rate; FACIT, Functional Assessment of Chronic Illness Therapy-Fatigue; GC, glucocorticoid; HAQ-DI, health assessment questionnaire disability index; IL-6, interleukin-6; MCS, Mental Component Summary scores and domains; MRA, magnetic resonance angiography; NA, not assessed; No, number; PCS, Physical Component Summary; PDN, prednisone; pts, patients; PtGA, Patient Global Assessment of Disease Activity; SF-36, 36-Item Short-Form Health Survey.
Shading: AEs reported without being considered part of the outcomes.
safety of treatment was summarized by extracting the data related to AEs.
