Abstract
Fetal exposure to prenatal stress can have significant consequences on short- and long-term health. Epigenetic mechanisms, especially DNA methylation (DNAm), are a possible process how these adverse environmental events could be biologically embedded. We evaluated candidate gene as well as epigenome-wide association studies associating prenatal stress and DNAm changes in peripheral tissues; however, most of these findings lack robust replication. Prenatal stress-associated epigenetic changes have also been linked to child health including internalizing problems, neurobehavioral outcomes and stress reactivity. Future studies should focus on refined measurement and definition of prenatal stress and its timing, ideally also incorporating genomic as well as longitudinal information. This will provide further opportunities to enhance our understanding of the biological embedding of prenatal stress exposure.
Keywords: DOHaD, Fetal programming, Prenatal stress, DNA methylation, Epigenetics
Introduction to fetal programming and prenatal stress
The concept of fetal programming
The fetal programming hypothesis, brought forward by Barker [3], originally postulated that undernutrition in the womb during pregnancy leads to reduced growth which causes a predisposition for cardiac and metabolic disorders in later adult life. This has been expanded into the more general Developmental Origins of Health and Disease (DOHaD) hypothesis, stating that exposure to adverse environmental events during sensitive periods of development and growth can have significant consequences on short- and long-term health [4, 14]. Short-term adaptations of the fetus to these adverse exposures include down-regulation of endocrine, metabolic or organ function to slow down growth rate and nutrient consumption [70]. This can have influences on gene expression, cell differentiation and proliferation leading to long-term, and often irreversible, changes in the structure of function of specific tissues and vital organs [5].
In fact, fetal programming has been described as predictive adaptive response (PAR) [30]: if the predicted environment is similar to the recent environment, the adaptation leads to an advantage. Hence, prenatal cues and developmental plasticity could influence the development of a phenotype that is adapted to the environmental conditions in later life, if this matches the early environment conditions [7]. The match/mismatch and PAR hypotheses both propose that moderate levels of early life stress can acquire resilience to renewed stress exposure later in life by preparing the offspring to better cope with a challenging adult environment [7, 83]. However, if there is a mismatch between predicted and recent environment, this may also increase risk for adverse outcomes in later life [36]. This can be illustrated with the following example: If a fetus develops in a nutrition-low environment, its metabolism adapts to this lack of nutrition. If, however, after birth, a lot of food is suddenly available, this can predispose the baby for overweight as its metabolism has not been trained for this mismatch. And in fact, children born with a low birth weight are at greater risk for developing metabolic syndrome later in life [26].
Definition of prenatal stress
Adverse environmental exposures involved in fetal programming are often described as prenatal stress, but how is prenatal stress actually defined? Prenatal stress, and stress itself, are very broad terms and different studies use different definitions. Generally speaking, stress presents if we are overwhelmed with the current situation and cannot adapt to it accordingly [16]. Several concepts of prenatal stress have been brought forward, reflecting the diversity of stressors which are present during pregnancy [18]. Psychosocial stressors, such as domestic violence and changes in personal life, may require adaptive coping by the affected individual [69]. These stressors can also affect pregnant women, and their offspring, but are not specific to pregnancy. On the other hand, pregnancy-specific stress refers to worries directly related to the pregnancy itself, i.e., concerns about outcome of prenatal screenings, or infant health and development [49]. Both, psychosocial and pregnancy-specific stress, can have strong effects on pregnancy and fetal development [18].
Following these definitions, a variety of different experiences have been explored in the prenatal context including adverse life events (e.g., trauma), depression, anxiety and contextual stress (e.g., financial difficulties), interpersonal risks (e.g., violence in a relationship) and risks due to parental characteristics (e.g., substance use) [15, 67]. Furthermore, also exposures to natural disasters during pregnancy have been studied as prenatal stressors [34, 45] and, very recently, the Covid-19 pandemic has added a whole new layer of complexity onto prenatal stress experiences [84].
Another very important point is that both the presence of a stressful event per se, i.e., the objective stress, and subjective stress, i.e., how stressful this event is perceived, need to be considered. In stress theory, three different types of stress have been described: response-based, stimulus-based and transactional-based stress [50]. In the response model, Selye [86] describes stress as physiological response pattern. In the stimulus-based definition of stress, stress is characterized as a stimulus such as traumatic events that cause certain reactions [38, 53]. This can also be defined as “objective” stress as it is based on the presence of a stressful event itself but does not take into account the intra-individual variability of how people react or are affected by the stressor. In the transactional-based definition of stress [48] on the other hand, the focus is not on the presence of the stressor itself but on the individual’s perception of this stressor. This could also be termed “subjective” stress. Any of the three types can also be found with regards to prenatal stress.
Given that fetal exposure to prenatal stress accounts for around 15% of the attributable risk for adverse mental health outcomes [29], it is important to study prenatal stress and how it affects the fetus. However, apart from the complexity of how prenatal stress is actually defined, also the exact processes which mediate fetal programming are not yet fully clear. Several possibilities have been suggested: (1) excessive exposure to glucocorticoids (GCs), (2) dysregulation of the hypothalamic–pituitary–adrenal (HPA)-axis, (3) irreversible changes in organ structure, (4) genetics, (5) epigenetic changes leading to altered gene expression and (6) cellular aging and intergenerational effects (see [51] for a review).
Scope of this review
To conclude, prenatal stress is a very important, but also very complex trait. Hence, we focus here on one specific layer of prenatal stress, namely how prenatal stress influences DNA methylation (DNAm), as one of the best understood epigenetic signatures in humans, and which consequences this might have on child health outcomes. We discuss mainly evidence from human studies but would like to point out that also animal studies support the hypothesis that prenatal stress could leave lasting signatures in DNAm (see [14] for a review). We conclude on what is important for future studies to further enlighten our understanding of prenatal stress, epigenetics and child outcome.
How does prenatal stress influence DNA methylation signatures in the offspring?
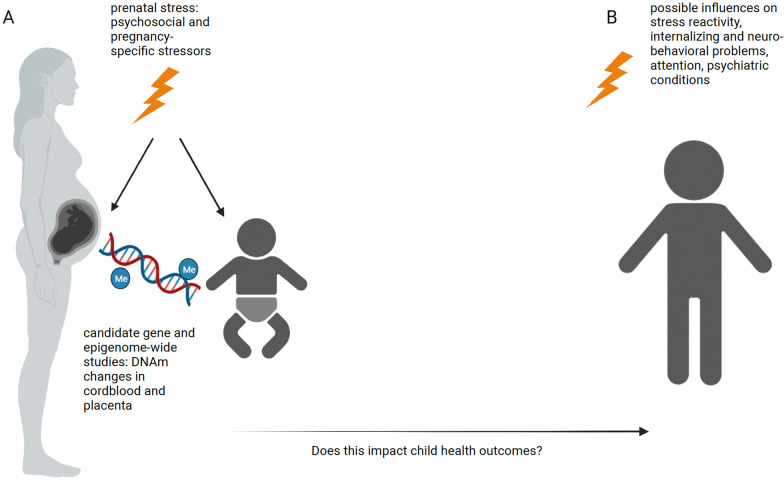
Epigenetic mechanisms, including DNAm, histone modifications and microRNAs, are one possible process of how prenatal stress could prime the offspring’s development (see Fig. 1A).
Fig. 1.
DNAm and prenatal stress. Prenatal stress can impact DNAm in perinatal tissues such as cordblood and placenta (A). This might have consequences on the child’s health including stress reactivity, neurobehavioral problems and psychiatric conditions (B). Created with BioRender.com
All of these epigenetic signatures could be influenced by prenatal stress and in turn regulate gene expression. Given that DNAm can be measured relatively straight-forward using large-scale methylation array techniques, it is the most widely studied and, so far, best understood, epigenetic mark in humans. As DNAm is tissue-specific [103], it should be mentioned that most studies assessed DNAm in buccal cells, saliva and blood, complemented by umbilical cord blood and placental tissue for the perinatal period. Information on prenatal stress is usually not available in human postmortem brain studies which rely on postmortem tissue from brain banks. In animal studies, a wider range of tissues, including brain, are accessible. This provided further insight into the effects of prenatal stress on epigenetic regulation [2, 14, 24, 31, 33, 62], but translating these findings to humans entails challenges.
Candidate gene studies
Studies in humans often focused on candidate genes involved in the HPA-axis (see Table 1). The HPA-axis is a main regulator of stress response, and it has been shown that maternal stress during pregnancy can lead to long-term effects on HPA-axis function and stress-related outcomes in the fetus [25, 54]. One of the most studied HPA-axis candidate genes is NR3C1 encoding the glucocorticoid receptor (GR), which itself is important for the negative feedback loop of the HPA-axis [22]. Interest in DNAm of NR3C1 was initiated by studies in rats: nr3c1 exon 1F methylation in the brain of offspring varied by maternal care and had relevance for GR-expression [104]. In human blood samples from children, DNAm of the NR3C1 promoter was associated with maternal prenatal stress experiences, such as intimate partner violence [79] and exposition to the Tutsi genocide [76]. In a meta-analysis of seven studies across different tissues, a significant correlation between prenatal maternal psychosocial stress and offspring DNAm at a CpG site located in the exon 1F of NR3C1 was observed [71, 72]. Furthermore, Turecki and Meaney [99] evaluated both animal and human studies and found an association of prenatal stress and increased methylation of NR3C1. Another systematic review assessed associations among maternal prenatal stress (defined as traumatic life events, stressful situations and perception of stressors, or the resulting phenotypes of stressors) and DNAm among commonly studied HPA-axis candidate genes (11BSHD2, OXTR, SLC6A4, CRH, CRHBP, FKBP5) in infants less than one year old [93]. The genes examined in this study are commonly considered candidates implicated in HPA-axis regulation. FKBP5 is involved in the termination of the stress response by regulating GR sensitivity [107]. 11BHSD2 is known as an important placental gene encoding an enzyme that catalyzes cortisol into cortisone and thus protects the fetus from excess cortisol exposure [85]. CRH and CRHBP are related to the production and release of cortisol by the pituitary gland as part of HPA-axis functioning [44]. The serotonin receptor gene SLC6A4 is implicated in the serotonergic modulation of the HPA-axis [77], and the oxytocin receptor gene, OXTR, is assumed to play a role for HPA-axis inhibition [35, 65]. The authors in [93] reported evidence for a link between prenatal stress and NR3C1 methylation, with more severe stressors showing stronger associations with infant DNAm as compared to stress-response phenotypes in the mothers. In another study by Kertes et al., widespread effects on DNAm in neonatal cord blood and placenta were reported among the CRH, CRHBP, NR3C1 and FKBP5 gene following prenatal traumatic war-related stress exposure, but the associations differed between tissues and were weaker for chronic stress compared to trauma [43].
Table 1.
Overview of referenced candidate genes studies investigating the association between DNAm and prenatal stress
| References | Candidate genes | Method DNAm | Sample Size | Exposure, exposure sample size and timing (if available) | Outcome and timing | Tissue | Age and sex (if available) | Result* |
|---|---|---|---|---|---|---|---|---|
| [43] | CRH, CRHBP, FKBP1, NR3C1 | 450 K array | 24 |
Prenatal psychosocial stress; 31% with 0–1 chronic stressors; 69% with 2–18 chronic stressors |
DNAm at birth | Cordblood,placenta |
Newborns; 54% male |
Effects on cord blood DNAm in CRH and NR3C1, effects on placenta DNAm in all four tested genes |
| [60] | FKBP5, HSD11B2, NR3C1 | 450 k array | 59 | Perceived maternal prenatal stress scale | DNAm at birth | Placenta |
Newborns; 49% male |
Higher perceived stress associated with higher DNAm |
| [63]a |
NR3C1, SLC6A4 |
Next-generation Sequencing (NGS) |
283 |
Pandemic lockdown; n = 81 in 1st trimester, n = 84 in 2nd trimester, n = 118 in 3rd trimester |
DNAm 6–24 h after birth |
Buccal | Newborns; 50% male | Higher methylation if exposed to pandemic lockdown during 2nd or 3rd trimester |
| [71, 72] | NR3C1 | Different |
977 (meta-analysis) |
Maternal chronic psychosocial stress during pregnancy | DNAm in children | Different | Newborns; one study included adolescents at mean age of 14 years | Significant correlation of DNAm and psychosocial stress |
| [76] |
NR3C1, NR3C2 |
Pyro-sequencing | 50 |
Exposure to Tusti genocide during pregnancy; n = 25 offspring of mothers exposed to Tutsi genocide during pregnancy, n = 25 unexposed |
DNAm in children | Blood | Adolescents aged 17–18 years, 48% male in exposed, 36% male in unexposed | Higher DNAm in NR3C1 in children whose mothers had been exposed |
| [78]a | SLC6A4 | NGS | 108 | Covid19-related prenatal stress score in 3rd trimester |
DNAm 6–24 h after birth |
Buccal |
Newborns; 49% male |
Hypermethylation in 3rd trimester |
| [79] | NR3C1 | NGS | 24 |
Intimate partner violence (IPV) in pregnancy; n = 8 with IPV, n = 16 without IPV |
DNAm in children | Blood |
Children 10–19 years; 33% male |
Positive relationship between DNAm and IPV in pregnancy |
| [93] |
11HSDB2, CRH, CRHBP, FKBP5, NR3C1, OXTR, SLC6A4 |
Different | Systematic review | Maternal stress in pregnancy | DNAm in infants | Different | Children aged below 12 months | Strongest association between most severe stressors and infant DNAm |
| [99] | NR3C1 | Different | Systematic review | Early life adversity, maternal psychosocial stress, parental stress | DNAm in offspring | Different | Children from birth to 18 years of age | Increased DNAm in exposed children |
| [106] | FKBP5 | Pyro-sequencing | 31 |
Holocaust experience, not only during pregnancy; n = 22 whose parents experienced holocaust, n = 9 whose parents did not experience holocaust |
DNAm in offspring | Blood |
Offspring; mean age 46–47 years; 27% male in exposed, 11% male in unexposed |
Lower FKBP5 DNAm in offspring of holocaust survivors |
Studies are listed in alphabetical order; studies are cross-sectional if not stated otherwise
*significance was defined differently throughout the studies
anote that both studies used overlapping datasets
DNAm changes in FKBP5 were also reported in blood samples of adult offspring of holocaust survivors [106]. Furthermore, perceived maternal stress was associated with increased placental DNAm of both HSD11B2 and FKBP5 and lower fetal heart rate-movement coupling, which is an indicator for fetal central nervous system development [60].
NR3C1 as well as SLC6A4 methylation has also been linked to maternal pandemic stress [63], interestingly a heightened sensitivity to epigenetic regulation could only be observed in mothers and their offspring exposed to lockdown in the second or third pregnancy trimester, but not in dyads exposed in the first trimester. In the same cohort and in accordance with this finding, Provenzi et al. [78] reported higher SLC6A4 methylation in children whose mothers reported pandemic stress in the last trimester.
Epigenome-wide association studies
As an alternative to candidate gene approaches, epigenome-wide association studies (EWAS) of DNAm, assessing the association between all CpG sites available on DNAm arrays and a trait of interest, have gained popularity during the last decade (see Fig. 1A and Table 2). Large sample sizes are necessary to detect the usually small effect sizes of single CpGs; hence, EWAS are usually conducted in large consortia, such as the Pregnancy And Childhood Epigenetics (PACE) consortium [27], where association results of single cohorts are pooled. While several epigenome-wide significant CpGs for maternal risk factors such as smoking (top hit in AHRR [42]) and maternal body mass index (BMI) at the beginning of pregnancy (top hit in VPR2 [91]) could be detected, no significant associations for maternal alcohol intake [90] and maternal anxiety [82] have been reported.
Table 2.
Overview of referenced epigenome-wide studies investigating the relationship between DNAm and prenatal stress
| References | Candidate genes | Method DNAm | Sample Size | Exposure, exposure sample size and timing (if available) | Outcome and timing | Tissue | Age and sex (if available) | Result* |
|---|---|---|---|---|---|---|---|---|
| [11] | EWAS | 450K array | 207 |
Maternal lifetime exposure to stress score assessed in 2nd trimester |
DNAm at birth |
Placenta |
Newborns; 52% male |
112 associated CpG sites (FDR 0.05) |
| [37] | EWAS | EPIC v1 array | 8 |
Maternal Covid-19 infection; n = 4 exposed, n = 4 unexposed |
DNAm in infants | Buccal | Infants aged 3 months; 88% male | 2,678 associated CpG sites (nominal p-value < 0.05) |
| [42] | EWAS | 450K array | 6685 |
Maternal smoking in pregnancy; n = 1,646 exposed to any smoking in pregnancy, n = 5,039 unexposed |
DNAm at birth |
Cordblood | Newborns | 6,073 associated CpG sites (FDR 0.05); 254 CpGs significantly associated with gene expression (FDR 0.05) |
| [47] | EWAS | EPIC v1 array | 44 |
Exposure to Covid-19 pandemic in utero; n = 32 exposed, n = 12 unexposed |
DNAm in infants | Buccal | Infants mean age 5 weeks; 56% male exposed; 58% male unexposed | 675 associated CpG sites (FDR 0.05) |
| [80] | EWAS | 450K array | 1740 | Prenatal maternal exposure score |
DNAm at birth |
Cordblood | Newborns; 51% males | No significantly associated CpG sites after multiple testing correction |
| [82] | EWAS |
450K array or EPIC v1 array |
7243 | Maternal anxiety score during pregnancy |
DNAm at birth |
Cordblood | Newborns | No significantly associated CpG sites after multiple testing correction |
| [89] | EWAS | EPIC v1 array | 114 | Prenatal maternal perceived stress |
DNAm at birth |
Saliva | Newborns; 54% male | One associated CpG site (FDR 0.05) |
| [91] | EWAS | 450K array | 9340 | Pre-Pregnancy maternal BMI |
DNAm at birth |
Cordblood | Newborns | 9,044 associated CpG sites (Bonferroni correction) |
| [90] | EWAS | 450K array | 3075 |
Alcohol consumption in pregnancy; n = 1147 exposed, n = 1928 unexposed |
DNAm at birth |
Cordblood | Newborns | No significantly associated CpG sites after multiple testing correction |
| [98] | EWAS | 450K array | 930 |
Maternal famine exposure during pregnancy; n = 73 exposed in weeks 1–10 of gestation, n = 123 exposed in weeks 11–20 of gestation, n = 143 exposed in weeks 21–30 of gestation, n = 128 exposed in weeks 31 up to delivery, n = 463 unexposed |
DNAm in adults | Blood | Adults; mean age 59; 46% male | 4 associated CpG sites with exposure in gestational weeks 1–10 after multiple testing correction, no associated CpG sites with exposure in later gestation |
| [100] | EWAS | EPIC v1 array | 16 |
Maternal Covid-19 infection; n = 8 exposed, n = 8 unexposed |
DNAm at birth | Cordblood | Newborns | 119 associated CpG sites (FDR 0.20) |
Studies are listed in alphabetical order; studies are cross-sectional if not stated otherwise
*significance was defined differently throughout the studies
In general, EWAS are often underpowered, effect sizes are usually small [93, 96] and, up to now, EWAS results for prenatal stress have been rather inconsistent. Rijlarrsdam et al. performed both an individual EWAS and a meta-analysis in cord blood, which revealed no epigenome-wide association between prenatal maternal stress and DNAm [80]. However, maternal life stress was associated with placental DNAm patterns of genes associated with endocytosis (i.e., SMAP1, ANKFY1), tight junctions (i.e., EPB41L4B), and metabolic pathways (i.e., INPP5E, EEF1B2), implicating roles for early embryo development [11]. Finally, a recent EWAS revealed associations between newborn epigenome-wide DNAm levels measured in saliva and chronic psychosocial stress experienced by the mother during pregnancy [89]. The associated genes including CSMD1, DAXX and ARL4D are relevant for neuronal, immune and endocrine homeostasis.
An EWAS on pandemic stress has been conducted confirming the role of NR3C1 as marker of prenatal stress [47]. Prenatal Covid-19 infection itself has been associated with DNAm changes in the offspring, albeit with very small sample sizes (8–16 samples): Hill et al. (R. A. [37] identified CpG sites in AFAP1 as well as in GAREM2 while [100] found associated CpGs in stress-response pathways.
EWAS also revealed that the timing of the prenatal stressor seems to be important. Using the quasi-experimental setting of the Dutch Famine, CpGs measured in adult whole blood were related to prenatal famine exposure during the first 10 weeks of gestation, but were not associated to stress exposure later in gestation. Interestingly, the identified CpGs were linked to genes with roles in growth, differentiation and metabolism (FAM150B, SLC38A2, PPAP2C and OSBPL5/MRGPRG) [98].
Epigenetic clocks
Since the development of the first epigenetic clocks in 2013 [32, 39], measurements of epigenetic aging constitute another method to investigate relationships between environmental influences and epigenetics (see Table 3). Epigenetic clocks aim to estimate the biological age of an individual from DNAm at specific CpGs that have previously been related to aging (see [40, 81] for an overview). Higher DNAm age as compared to chronological age is referred to as ‘age acceleration,’ lower DNAm age as compared to chronological age is referred to as ‘age deceleration,’ and this ticking rate of an epigenetic clock has been associated with age-related diseases [105]. Although there are no human studies to date which focus specifically on prenatal stress and epigenetic aging in fetal tissues, prenatal adverse environment was linked to epigenetic age deceleration in cordblood [73]. Moreover, antenatal depressive symptoms were related to age deceleration in cordblood [94], but prenatal selective serotonin reuptake inhibitors (SSRIs) use could significantly contribute to this association [57]. In addition, prenatal maternal anxiety predicted child epigenetic age acceleration, however only in males [55]. This underscores that sex can be an important modifier in DNAm studies. Furthermore, we observed that gestational epigenetic aging is not related to more favorable or unfavorable factors in a clear direction in placenta and cord blood, and epigenetic aging patterns are tissue specific [23].
Table 3.
Overview of referenced studies investigating the association between epigenetic age and prenatal stress
| References | Candidate genes | Method DNAm | Sample Size | Exposure, exposure sample size and timing (if available) | Outcome and timing | Tissue | Age and sex (if available) | Result* |
|---|---|---|---|---|---|---|---|---|
| [55] | Epigenetic age, longitudinal study | EPIC v1 array | 505 | Prenatal maternal anxiety, externalizing problems in offspring |
DNAm in infants and children |
Buccal | Children; aged 3, 9, 48 months, 6 and 10 years | Prenatal maternal anxiety associated with age acceleration; age acceleration associated with externalizing problems in boys |
| [73] | Epigenetic age | EPIC v1 array | 60 | Cerebroplacental ratio (CPR) in 3rd trimester |
DNAm at birth |
Cordblood | Newborns | Decreased epigenetic age acceleration in children associated with decreased CPR |
| [94] | Epigenetic age | 450K array | 407 | Antenatal depressive symptoms |
DNAm at birth |
Cordblood | Newborns; 53% males | Decreased epigenetic age acceleration in children associated with maternal depressive symptoms |
Studies are listed in alphabetical order; studies are cross-sectional if not stated otherwise
*significance was defined differently throughout the studies
Are prenatal stress-associated epigenetic changes related to child health outcomes?
In the previous section, we discussed associations between prenatal stress and epigenetic changes, focusing on DNAm. These epigenetic alterations were often related to HPA-axis function and have the potential to prime the child, for example regarding long-term stress responsiveness. Hence, prenatal stress may increase the risk for altered physiological and psychiatric outcomes in the offspring through epigenetic mechanisms (see Fig. 1B and Table 4).
Table 4.
Overview of referenced studies investigating the association between DNAm and child outcome
| References | Candidate genes | Method DNAm | Sample Size | Exposure, exposure sample size and timing (if available) | Outcome and timing | Tissue | Age and sex (if available) | Result* |
|---|---|---|---|---|---|---|---|---|
| [1] |
HSD11B2, NR3C1 |
Pyro-sequencing | 372 | Infant neurobehavior (NICU Network Neurobehavioral Scales (NNNS)) |
DNAm at birth |
Placenta | Newborns; 50% male | Low NR3C1 DNAm and high HSD11B2 DNAm associated with lower excitability scores; high NR3C1 DNAm and low HSD11B2 DNAm associated with asymmetrical reflexes; high DNAm associated with higher habituation scores |
| [17] |
HSD11B2, NR3C1 |
Pyro-sequencing | 482 | Prenatal maternal anxiety and depression | DNAm and internalizing problems | Placenta | Newborns; 48% male | Positive correlation between NR3C1 DNAm and internalizing problems |
| [68] | NR3C1 | Pyro-sequencing | 82 |
Depressed/ anxious mood in 3rd trimester |
DNAm in newborns and cortisol response at 3 months | Cordblood | Newborns; 44% male | Hypermethylation in NR3C1 which was also associated with increased HPA stress responsiveness at 3 months |
| [13]a | Candidate genes from established Type-1 and -2 diabetes mellitus pathways | 450K array | 31 | Maternal stress during exposure to Quebec ice storm |
DNAm, child BMI |
Blood | Children; mean age 13 years | Significant negative mediation of DNAm of the effect of objective prenatal maternal stress on central adiposity and BMI |
| [12]a | Candidate genes from established Type-1 and -2 diabetes mellitus pathways | 450K array | 30 | Maternal stress during exposure to Quebec ice storm |
DNAm, C-peptide secretion in response to an oral glucose tolerance test |
Blood | Children; mean age 13 years | Significant mediation of DNAm of the relationship between maternal cognitive appraisal of a natural disaster in pregnancy and C-peptide production in adolescent offspring |
| [46] | FKBP5 | Pyro-sequencing | 76 |
Childhood trauma; n = 30 exposed, n = 46 unexposed; glucocortiocoid receptor sensitivity |
DNAm in adults | Blood | Adults; mean age 41 years | Allele-specific, childhood trauma–dependent DNA demethylation in functional glucocorticoid response elements of FKBP5 linked to dysregulation of the stress hormone system and a global effect on the function of immune cells and brain areas associated with stress regulation |
| [52] | HSD11B2 | Pyro-sequencing | 185 | NNNS | DNAm | Placenta | Newborns; 45% male | Increasing DNAm associated with reduced scores of quality of movement; increased DNAm associated with lower expression of HSD11B2 |
| [74] | FKBP5 | Pyro-sequencing | 509 | NNNS | DNAm | Placenta | Newborns; 49% male | Infants in the highest quartile of FKBP5 DNAm with increased risk of NNNS high arousal compared to infants in the lowest quartile |
| [75] |
ADCYAP1R1, FKBP5, HSD11B2, NR3C1 |
Pyro-sequencing | 537 | NNNS | DNAm | Placenta | Newborns; 49% male | DNAm patterns of glucocorticoid response genes associated with adaption to postnatal stress among healthy populations of infants exposed to low-to-moderate prenatal stress |
Studies are listed in alphabetical order; studies are cross-sectional if not stated otherwise
*significance was defined differently throughout the studies
anote that both studies used overlapping datasets
Again, the glucocorticoid receptor gene is the candidate gene with the strongest evidence. For example, increased DNAm at a CpG in NR3C1 in cord blood after prenatal exposure to maternal depressed mood was related to HPA-axis stress reactivity of the child at three months of age [68]. Using placental samples, Conradt et al. [17] identified a relationship between NR3C1 DNAm with infant quality of movement and attention. In a systematic review on DNAm of the glucocorticoid receptor gene the link between early life adversity, hypermethylation and impaired HPA-axis functioning, which may predispose individuals to psychiatric conditions, was supported [71, 72]. Additionally, it was suggested that placental DNAm of NR3C1 and HSD11B2 may jointly influence distinct domains of newborn neurobehavior [1].
Effects of maternal reports of depression or anxiety during pregnancy were related to neurobehavioral outcomes in the newborns and placental DNAm in NR3C1 and 11BHSD2 [17, 52] as well as in FKBP5 [74]. Moreover, Klengel et al. [46] provide evidence for FKBP5 DNAm mediating the combined effect of early trauma exposure and a genetic polymorphism in FKBP5 on the risk of developing stress-related psychiatric disorders using peripheral blood in adults. In another study, placental DNAm patterns of four candidate glucocorticoid response genes (NR3C1, HSD11B2, FKBP5 and ADCYAP1R1) were related to risk of neurobehavioral adversity, i.e., different ability to adapt to stress in the postnatal environment, in a healthy population of infants [75].
Studies on natural disasters also support the mediating role of DNAm on the association between prenatal maternal stress exposures and the child’s metabolic outcomes and immune system: Cao-Lei et al. [13] report that DNAm of selected genes in the type 2 diabetes pathway mediated the association of objective prenatal maternal stress due to the Quebec ice storm and adiposity in teenage children. Adding to this, the relationship between objective hardship experienced by the mothers and C-peptide secretion in adolescent children was also mediated by blood DNAm [12].
This relationship between stress and trauma exposure of parents and a greater risk of psychopathology among children, accompanied by epigenetic modification, is supported by the concept of intergenerational transmission brought forward by Bowers et al. [10], although the etiology of effects in humans is impeded by methodological constraints. Congruently, Monk et al. [61] state that there is evidence for an association between maternal prenatal distress and both fetal and infant developmental trajectories where epigenetic mechanisms may serve as a biological link mediating these effects, but influences of the postnatal environment must be carefully considered to further elucidate these pathways.
The postnatal environment is not only important to consider on a methodological level as a confounder, but should also be taken into account to understand the demonstrated relationships between prenatal stress and child outcomes from an evolutionary perspective and including the match/mismatch hypothesis. Epigenetic mechanisms can fine-tune gene expression to allow the organism to adapt to the environment [6]. Accordingly, there should not be a one-sided perspective of stress causing vulnerability for diseases. There is also an adaptive side of epigenetic modifications following prenatal exposures, preparing the developing organism for later environment. For example, a study by Zhang et al. [108] supports the idea that a moderate amount of normative prenatal stress may buffer the impact of traumatic prenatal stress, in this case caused by Superstorm Sandy, on placental gene expression. And Serpeloni et al. [87] showed that children had better mental health, when pre- and postnatal environment matched, i.e., there were less psychiatric problems among children who experienced intimate partner violence prenatally and postnatally compared to children who experienced this only postnatally. These studies demonstrate the possibility of adaptive programming following early experiences. In summary, changes after prenatal exposure to stress could favor developmental adjustment accomplished by epigenetic alterations which should not only be seen as detrimental, but also evolutionarily adaptive to some extent. Finally, individual differences in the sensitivity to early programming should be considered [64].
Summary
In conclusion, there is some evidence from candidate gene studies as well as from EWAS that prenatal stress is associated with changes in DNAm and that these changes could also have impact on child health outcomes. When evaluating these findings, it is essential to be aware of the limitations and generalizability of described associations. First of all, DNAm can vary by ancestry [41] and also the genome plays an important role—allele-specific DNAm patterns [58, 92] as well as meQTLs (methylation quantitative trait loci, [59]) have been reported. By design, EWAS focus on main effects of specific traits, such as prenatal stress, on DNAm but do not include gene-environment interactions (GxE). However, the child’s genetic susceptibility might be of importance as it has been shown that GxE effects explain the majority of variation of DNAm in the pre- and postnatal context [19, 20, 97]. Moreover, child sex and age have been related to both DNAm [9] and the stress exposure itself [56, 95] and stratified analyses might be beneficial to uncover DNAm signatures in subgroups.
Most epigenetic studies focused on main effects of prenatal stress on DNAm. However, it should be noted, that other important factors need to be taken into account to disentangle the path from prenatal stress to DNAm and outcome in the offspring. On the one hand, prenatal stress and its consequences on maternal physiology themselves can be mediated by factors such as diet, sleep or exercise [21]. On the other hand, effects of prenatal stress on the offspring can be enhanced by postnatal stress but also be reversed by postnatal supportive environment [66]. Only few studies have looked into this relationship on the level of DNAm.
Moreover, not only the type of stressor but also the timing of the stress exposure during pregnancy can play an important role, considering different susceptible periods during fetal development (Van den [102].
Tissue specificity is another major challenge, for example relevant whenever translating animal research to humans and explaining incongruencies between results of different studies. Epigenetic mechanisms leading to differential gene expression are a central part of cell development and differentiation, and thus they vary to some extent between developmental stages and tissues [8]. Hence, findings based on DNAm patterns in one tissue cannot be directly transferred to DNAm effects in other tissues.
Finally, only looking into DNAm depicts only a limited picture as DNAm itself can have further consequences on gene expression. Of the investigated studies, only one reported a significant association of identified CpG sites with gene expression [52] and often studies did not evaluate gene expression in their samples at all.
Implications for future studies
It has been shown, both in animal and human studies, that prenatal stress can be related to epigenetic changes, and the period from conception to early childhood seems to be the most critical one [51]. However, robust, replicated findings are still scarce. What could be the “mission” for future studies? Four main points are important here: 1) the timing and intensity of the stressor, 2) the definition of the stressor itself, 3) in which tissue an effect was observed and 4) a longitudinal study design. The prenatal period is a dynamic and developmentally important phase making it very interesting but also very complex to study. Most studies used a cross-sectional design where the presence of a prenatal stressor was associated to DNAm levels at one specific timepoint, usually in perinatal tissues at birth. To be able to reflect these dynamic changes in the prenatal period, however, ideally, longitudinal measurement should be assessed. This refers to the timing and course of prenatal stress itself. Furthermore, if prenatal stress is associated with epigenetic changes, a very important question is if this also leads to long-lasting changes in DNAm signatures and in child development or might be moderated by postnatal environment. Hence, not only follow-up during pregnancy but also after birth is essential, including not only repeated phenotypes on child outcome but also repeated DNAm measures, and ideally also gene expression, throughout childhood and beyond.
Furthermore, it still remains inconclusive if the existence of any prenatal stressor or rather of specific prenatal stressors are implicated in DNAm changes. The operationalization of prenatal stress must therefore be taken into account. The definition of stress varies between studies and different stressors could have distinct effects. Hence, refined measurement and definition of prenatal stress and its timing, ideally also incorporating genomic information, will provide further opportunities to enhance our understanding of the biological embedding of prenatal stress exposures.
All of these points potentially contribute to heterogeneous results reported in the literature and are momentous when interpreting individual findings. Indeed, the role of the reported associations as potential causal mechanisms is mostly not clear, especially in humans, where we rely on peripheral tissues and often retrospective designs. Importantly however, they could still serve as biomarkers for those processes that in the end lead to disease trajectories.
To date, most studies have been focusing on DNAm in cord blood, while only some used placental tissue. The placenta is heterogeneous and challenging to study, however, given its importance for fetal development [28] and its possible role in early neurodevelopment [101], it should not be neglected and future studies should also look more closely into placental DNAm.
Given that the mentioned limitations are accounted for as far as possible, DNAm biomarkers have the potential to improve our understanding of prenatal programming. Enlightening these processes and their long-term consequences will also enhance our knowledge on children’s health care [88].
Acknowledgements
Not applicable.
Abbreviations
- BMI
Body mass index
- DNAm
DNA methylation
- DOHaD
Developmental origins of health and disease
- EWAS
Epigenome-wide association studies
- GC
Glucocorticoid
- GR
Glucocorticoid receptor
- HPA-axis
Hypothalamic–pituitary–adrenal-axis
- PACE consortium
Pregnancy and childhood epigenetics consortium
- PAR
Predictive adaptive response
- SSRI
Selective serotonin reuptake inhibitor
- meQTL
Methylation quantitative trait locus
Author contributions
LR searched the literature, wrote the manuscript, and approved the final version of the manuscript. DC searched the literature, wrote the manuscript, and approved the final version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. Linda Dieckmann was supported by the Add-on Fellowship of the Joachim Herz Foundation.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Appleton AA, Lester BM, Armstrong DA, Lesseur C, Marsit CJ. Examining the joint contribution of placental NR3C1 and HSD11B2 methylation for infant neurobehavior. Psychoneuroendocrinology. 2015;52:32–42. doi: 10.1016/j.psyneuen.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babenko O, Kovalchuk I, Metz GA. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev. 2015;48:70–91. doi: 10.1016/j.neubiorev.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ. In utero programming of chronic disease. Clin Sci (Lond) 1998;95(2):115–128. doi: 10.1042/cs0950115. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ, Forsen T, Uutela A, Osmond C, Eriksson JG. Size at birth and resilience to effects of poor living conditions in adult life: longitudinal study. BMJ. 2001;323(7324):1273–1276. doi: 10.1136/bmj.323.7324.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: implications for research and public health. Environ Health. 2012;11:42. doi: 10.1186/1476-069X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bateson P, Gluckman P, Hanson M. The biology of developmental plasticity and the predictive adaptive response hypothesis. J Physiol. 2014;592(11):2357–2368. doi: 10.1113/jphysiol.2014.271460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogdanovic O, van Heeringen SJ, Veenstra GJ. The epigenome in early vertebrate development. Genesis. 2012;50(3):192–206. doi: 10.1002/dvg.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boks MP, Derks EM, Weisenberger DJ, Strengman E, Janson E, Sommer IE, Ophoff RA. The relationship of DNA methylation with age, gender and genotype in twins and healthy controls. PLoS ONE. 2009;4(8):e6767. doi: 10.1371/journal.pone.0006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowers ME, Yehuda R. Intergenerational transmission of stress in humans. Neuropsychopharmacology. 2016;41(1):232–244. doi: 10.1038/npp.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunst KJ, Tignor N, Just A, Liu Z, Lin X, Hacker MR, Wright RJ. Cumulative lifetime maternal stress and epigenome-wide placental DNA methylation in the PRISM cohort. Epigenetics. 2018;13(6):665–681. doi: 10.1080/15592294.2018.1497387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao-Lei L, Dancause KN, Elgbeili G, Laplante DP, Szyf M, King S. DNA methylation mediates the effect of maternal cognitive appraisal of a disaster in pregnancy on the child's C-peptide secretion in adolescence: Project Ice Storm. PLoS ONE. 2018;13(2):e0192199. doi: 10.1371/journal.pone.0192199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao-Lei L, Dancause KN, Elgbeili G, Massart R, Szyf M, Liu A, King S. DNA methylation mediates the impact of exposure to prenatal maternal stress on BMI and central adiposity in children at age 13(1/2) years: project ice storm. Epigenetics. 2015;10(8):749–761. doi: 10.1080/15592294.2015.1063771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao-Lei L, de Rooij SR, King S, Matthews SG, Metz GAS, Roseboom TJ, Szyf M. Prenatal stress and epigenetics. Neurosci Biobehav Rev. 2020;117:198–210. doi: 10.1016/j.neubiorev.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Cecil CA, Lysenko LJ, Jaffee SR, Pingault JB, Smith RG, Relton CL, Barker ED. Environmental risk, oxytocin receptor gene (OXTR) methylation and youth callous-unemotional traits: a 13-year longitudinal study. Mol Psychiatry. 2014;19(10):1071–1077. doi: 10.1038/mp.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen S, Kessler RC, Gordon LU. Strategies for measuring stress in studies of psychiatric and physical disorders. In: Cohen S, Kessler RC, Gordon LU, editors. Measuring stress: A guide for health and social scientists. New York: Oxford University Press; 1995. pp. 3–26. [Google Scholar]
- 17.Conradt E, Lester BM, Appleton AA, Armstrong DA, Marsit CJ. The roles of DNA methylation of NR3C1 and 11beta-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics. 2013;8(12):1321–1329. doi: 10.4161/epi.26634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coussons-Read ME. Effects of prenatal stress on pregnancy and human development: mechanisms and pathways. Obstet Med. 2013;6(2):52–57. doi: 10.1177/1753495X12473751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czamara D, Eraslan G, Page CM, Lahti J, Lahti-Pulkkinen M, Hamalainen E, Binder EB. Integrated analysis of environmental and genetic influences on cord blood DNA methylation in new-borns. Nat Commun. 2019;10(1):2548. doi: 10.1038/s41467-019-10461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Czamara D, Tissink E, Tuhkanen J, Martins J, Awaloff Y, Drake AJ, Binder EB. Combined effects of genotype and childhood adversity shape variability of DNA methylation across age. Transl Psychiatry. 2021;11(1):88. doi: 10.1038/s41398-020-01147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Weerth C. Prenatal stress and the development of psychopathology: Lifestyle behaviors as a fundamental part of the puzzle. Dev Psychopathol. 2018;30(3):1129–1144. doi: 10.1017/S0954579418000494. [DOI] [PubMed] [Google Scholar]
- 22.DeRijk RH, Schaaf M, de Kloet ER. Glucocorticoid receptor variants: clinical implications. J Steroid Biochem Mol Biol. 2002;81(2):103–122. doi: 10.1016/s0960-0760(02)00062-6. [DOI] [PubMed] [Google Scholar]
- 23.Dieckmann L, Lahti-Pulkkinen M, Kvist T, Lahti J, DeWitt PE, Cruceanu C, Czamara D. Characteristics of epigenetic aging across gestational and perinatal tissues. Clin Epigenetics. 2021;13(1):97. doi: 10.1186/s13148-021-01080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong E, Pandey SC. Prenatal stress induced chromatin remodeling and risk of psychopathology in adulthood. Int Rev Neurobiol. 2021;156:185–215. doi: 10.1016/bs.irn.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duthie L, Reynolds RM. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: influences on maternal and fetal outcomes. Neuroendocrinology. 2013;98(2):106–115. doi: 10.1159/000354702. [DOI] [PubMed] [Google Scholar]
- 26.Eriksson JG, Forsen T, Tuomilehto J, Winter PD, Osmond C, Barker DJ. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ. 1999;318(7181):427–431. doi: 10.1136/bmj.318.7181.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felix JF, Joubert BR, Baccarelli AA, Sharp GC, Almqvist C, Annesi-Maesano I, London SJ. Cohort profile: pregnancy and childhood epigenetics (PACE) consortium. Int J Epidemiol. 2018;47(1):22–23u. doi: 10.1093/ije/dyx190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garnica AD, Chan WY. The role of the placenta in fetal nutrition and growth. J Am Coll Nutr. 1996;15(3):206–222. doi: 10.1080/07315724.1996.10718591. [DOI] [PubMed] [Google Scholar]
- 29.Glover V. Prenatal stress and its effects on the fetus and the child: possible underlying biological mechanisms. Adv Neurobiol. 2015;10:269–283. doi: 10.1007/978-1-4939-1372-5_13. [DOI] [PubMed] [Google Scholar]
- 30.Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends Ecol Evol. 2005;20(10):527–533. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Goyal D, Limesand SW, Goyal R. Epigenetic responses and the developmental origins of health and disease. J Endocrinol. 2019;242(1):T105–T119. doi: 10.1530/JOE-19-0009. [DOI] [PubMed] [Google Scholar]
- 32.Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Zhang K. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49(2):359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haq SU, Bhat UA, Kumar A. Prenatal stress effects on offspring brain and behavior: Mediators, alterations and dysregulated epigenetic mechanisms. J Biosci. 2021;46:1–16. doi: 10.1007/s12038-021-00153-7. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins G, Gullam J, Belluscio L. The effect of a major earthquake experienced during the first trimester of pregnancy on the risk of preterm birth. Aust N Z J Obstet Gynaecol. 2019;59(1):82–88. doi: 10.1111/ajo.12797. [DOI] [PubMed] [Google Scholar]
- 35.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54(12):1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 36.Hill J, Pickles A, Wright N, Braithwaite E, Sharp H. Predictions of children's emotionality from evolutionary and epigenetic hypotheses. Sci Rep. 2019;9(1):2519. doi: 10.1038/s41598-019-39513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hill RA, Gibbons A, Han U, Suwakulsiri W, Taseska A, Hammet F, Sundram S. Maternal SARS-CoV-2 exposure alters infant DNA methylation. Brain Behav Immun Health. 2023;27:100572. doi: 10.1016/j.bbih.2022.100572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holmes TH, Rahe RH. The Social Readjustment Rating Scale. J Psychosom Res. 1967;11(2):213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 39.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19(6):371–384. doi: 10.1038/s41576-018-0004-3. [DOI] [PubMed] [Google Scholar]
- 41.Husquin LT, Rotival M, Fagny M, Quach H, Zidane N, McEwen LM, Quintana-Murci L. Exploring the genetic basis of human population differences in DNA methylation and their causal impact on immune gene regulation. Genome Biol. 2018;19(1):222. doi: 10.1186/s13059-018-1601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joubert BR, Felix JF, Yousefi P, Bakulski KM, Just AC, Breton C, London SJ. DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am J Hum Genet. 2016;98(4):680–696. doi: 10.1016/j.ajhg.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kertes DA, Kamin HS, Hughes DA, Rodney NC, Bhatt S, Mulligan CJ. Prenatal maternal stress predicts methylation of genes regulating the hypothalamic-pituitary-adrenocortical system in mothers and newborns in the democratic Republic of Congo. Child Dev. 2016;87(1):61–72. doi: 10.1111/cdev.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ketchesin KD, Stinnett GS, Seasholtz AF. Corticotropin-releasing hormone-binding protein and stress: from invertebrates to humans. Stress. 2017;20(5):449–464. doi: 10.1080/10253890.2017.1322575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.King S, Dancause K, Turcotte-Tremblay AM, Veru F, Laplante DP. Using natural disasters to study the effects of prenatal maternal stress on child health and development. Birth Defects Res C Embryo Today. 2012;96(4):273–288. doi: 10.1002/bdrc.21026. [DOI] [PubMed] [Google Scholar]
- 46.Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Binder EB. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16(1):33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kocher K, Bhattacharya S, Niforatos-Andescavage N, Almalvez M, Henderson D, Vilain E, Delot EC. Genome-wide neonatal epigenetic changes associated with maternal exposure to the COVID-19 pandemic. BMC Med Genomics. 2023;16(1):268. doi: 10.1186/s12920-023-01707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lazarus RS. Psychological stress and the coping process. New York: McGraw-Hill; 1966. [Google Scholar]
- 49.Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol. 2008;27(5):604–615. doi: 10.1037/a0013242. [DOI] [PubMed] [Google Scholar]
- 50.Lyon BL. Stress, coping, and health: A conceptual overview (update) In: Rice VH, editor. Handbook of stress, coping, and health: Implications for nursing research, theory, and practict. Sage Publications Inc; 2012. pp. 2–20. [Google Scholar]
- 51.Mandy M, Nyirenda M. Developmental Origins of Health and Disease: the relevance to developing nations. Int Health. 2018;10(2):66–70. doi: 10.1093/inthealth/ihy006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marsit CJ, Maccani MA, Padbury JF, Lester BM. Placental 11-beta hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome. PLoS ONE. 2012;7(3):e33794. doi: 10.1371/journal.pone.0033794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masuda M, Holmes TH. Magnitude estimations of social readjustments. J Psychosom Res. 1967;11(2):219–225. doi: 10.1016/0022-3999(67)90011-6. [DOI] [PubMed] [Google Scholar]
- 54.Matthews SG, McGowan PO. Developmental programming of the HPA axis and related behaviours: epigenetic mechanisms. J Endocrinol. 2019;242(1):T69–T79. doi: 10.1530/JOE-19-0057. [DOI] [PubMed] [Google Scholar]
- 55.McGill MG, Pokhvisneva I, Clappison AS, McEwen LM, Beijers R, Tollenaar MS, O'Donnell KJ. Maternal prenatal anxiety and the fetal origins of epigenetic aging. Biol Psychiatry. 2022;91(3):303–312. doi: 10.1016/j.biopsych.2021.07.025. [DOI] [PubMed] [Google Scholar]
- 56.McGowan PO, Matthews SG. Prenatal stress, glucocorticoids, and developmental programming of the stress response. Endocrinology. 2018;159(1):69–82. doi: 10.1210/en.2017-00896. [DOI] [PubMed] [Google Scholar]
- 57.McKenna BG, Hendrix CL, Brennan PA, Smith AK, Stowe ZN, Newport DJ, Knight AK. Maternal prenatal depression and epigenetic age deceleration: testing potentially confounding effects of prenatal stress and SSRI use. Epigenetics. 2021;16(3):327–337. doi: 10.1080/15592294.2020.1795604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meaburn EL, Schalkwyk LC, Mill J. Allele-specific methylation in the human genome: implications for genetic studies of complex disease. Epigenetics. 2010;5(7):578–582. doi: 10.4161/epi.5.7.12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Min JL, Hemani G, Hannon E, Dekkers KF, Castillo-Fernandez J, Luijk R, Relton CL. Genomic and phenotypic insights from an atlas of genetic effects on DNA methylation. Nat Genet. 2021;53(9):1311–1321. doi: 10.1038/s41588-021-00923-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monk C, Feng T, Lee S, Krupska I, Champagne FA, Tycko B. Distress during pregnancy: epigenetic regulation of placenta glucocorticoid-related genes and fetal neurobehavior. Am J Psychiatry. 2016;173(7):705–713. doi: 10.1176/appi.ajp.2015.15091171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monk C, Spicer J, Champagne FA. Linking prenatal maternal adversity to developmental outcomes in infants: the role of epigenetic pathways. Dev Psychopathol. 2012;24(4):1361–1376. doi: 10.1017/S0954579412000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12(12):1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- 63.Nazzari S, Grumi S, Mambretti F, Villa M, Giorda R, Provenzi L, MCS Group Maternal and infant NR3C1 and SLC6A4 epigenetic signatures of the COVID-19 pandemic lockdown: when timing matters. Transl Psychiatry. 2022;12(1):386. doi: 10.1038/s41398-022-02160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nederhof E, Schmidt MV. Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol Behav. 2012;106(5):691–700. doi: 10.1016/j.physbeh.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 65.Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J Neuroendocrinol. 2000;12(3):235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- 66.Nolvi S, Merz EC, Kataja EL, Parsons CE. Prenatal stress and the developing brain: postnatal environments promoting resilience. Biol Psychiatry. 2023;93(10):942–952. doi: 10.1016/j.biopsych.2022.11.023. [DOI] [PubMed] [Google Scholar]
- 67.O'Donnell K, O'Connor TG, Glover V. Prenatal stress and neurodevelopment of the child: focus on the HPA axis and role of the placenta. Dev Neurosci. 2009;31(4):285–292. doi: 10.1159/000216539. [DOI] [PubMed] [Google Scholar]
- 68.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3(2):97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 69.Orr ST, James SA, Casper R. Psychosocial stressors and low birth weight: development of a questionnaire. J Dev Behav Pediatr. 1992;13(5):343–347. doi: 10.1097/00004703-199210010-00005. [DOI] [PubMed] [Google Scholar]
- 70.Osmond C, Barker DJ, Winter PD, Fall CH, Simmonds SJ. Early growth and death from cardiovascular disease in women. BMJ. 1993;307(6918):1519–1524. doi: 10.1136/bmj.307.6918.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palma-Gudiel H, Cordova-Palomera A, Eixarch E, Deuschle M, Fananas L. Maternal psychosocial stress during pregnancy alters the epigenetic signature of the glucocorticoid receptor gene promoter in their offspring: a meta-analysis. Epigenetics. 2015;10(10):893–902. doi: 10.1080/15592294.2015.1088630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palma-Gudiel H, Cordova-Palomera A, Leza JC, Fananas L. Glucocorticoid receptor gene (NR3C1) methylation processes as mediators of early adversity in stress-related disorders causality: a critical review. Neurosci Biobehav Rev. 2015;55:520–535. doi: 10.1016/j.neubiorev.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 73.Palma-Gudiel H, Eixarch E, Crispi F, Moran S, Zannas AS, Fananas L. Prenatal adverse environment is associated with epigenetic age deceleration at birth and hypomethylation at the hypoxia-responsive EP300 gene. Clin Epigenetics. 2019;11(1):73. doi: 10.1186/s13148-019-0674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paquette AG, Lester BM, Koestler DC, Lesseur C, Armstrong DA, Marsit CJ. Placental FKBP5 genetic and epigenetic variation is associated with infant neurobehavioral outcomes in the RICHS cohort. PLoS ONE. 2014;9(8):e104913. doi: 10.1371/journal.pone.0104913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paquette AG, Lester BM, Lesseur C, Armstrong DA, Guerin DJ, Appleton AA, Marsit CJ. Placental epigenetic patterning of glucocorticoid response genes is associated with infant neurodevelopment. Epigenomics. 2015;7(5):767–779. doi: 10.2217/epi.15.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perroud N, Rutembesa E, Paoloni-Giacobino A, Mutabaruka J, Mutesa L, Stenz L, Karege F. The Tutsi genocide and transgenerational transmission of maternal stress: epigenetics and biology of the HPA axis. World J Biol Psychiatry. 2014;15(4):334–345. doi: 10.3109/15622975.2013.866693. [DOI] [PubMed] [Google Scholar]
- 77.Porter RJ, Gallagher P, Watson S, Young AH. Corticosteroid-serotonin interactions in depression: a review of the human evidence. Psychopharmacology. 2004;173(1–2):1–17. doi: 10.1007/s00213-004-1774-1. [DOI] [PubMed] [Google Scholar]
- 78.Provenzi L, Mambretti F, Villa M, Grumi S, Citterio A, Bertazzoli E, Borgatti R. Hidden pandemic: COVID-19-related stress, SLC6A4 methylation, and infants' temperament at 3 months. Sci Rep. 2021;11(1):15658. doi: 10.1038/s41598-021-95053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Radtke KM, Ruf M, Gunter HM, Dohrmann K, Schauer M, Meyer A, Elbert T. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl Psychiatry. 2011;1(7):e21. doi: 10.1038/tp.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rijlaarsdam J, Pappa I, Walton E, Bakermans-Kranenburg MJ, Mileva-Seitz VR, Rippe RC, van, I. M. H. An epigenome-wide association meta-analysis of prenatal maternal stress in neonates: A model approach for replication. Epigenetics. 2016;11(2):140–149. doi: 10.1080/15592294.2016.1145329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ryan CP. "Epigenetic clocks": Theory and applications in human biology. Am J Hum Biol. 2021;33(3):e23488. doi: 10.1002/ajhb.23488. [DOI] [PubMed] [Google Scholar]
- 82.Sammallahti S, Cortes Hidalgo AP, Tuominen S, Malmberg A, Mulder RH, Brunst KJ, Lahti J. Maternal anxiety during pregnancy and newborn epigenome-wide DNA methylation. Mol Psychiatry. 2021;26(6):1832–1845. doi: 10.1038/s41380-020-00976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Santarelli S, Zimmermann C, Kalideris G, Lesuis SL, Arloth J, Uribe A, Schmidt MV. An adverse early life environment can enhance stress resilience in adulthood. Psychoneuroendocrinology. 2017;78:213–221. doi: 10.1016/j.psyneuen.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 84.Schoenmakers S, Verweij EJJ, Beijers R, Bijma HH, Been JV, Steegers-Theunissen RPM, Steegers EAP. The Impact of Maternal Prenatal Stress Related to the COVID-19 Pandemic during the First 1000 Days: A Historical Perspective. Int J Environ Res Public Health. 2022 doi: 10.3390/ijerph19084710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schoof E, Girstl M, Frobenius W, Kirschbaum M, Repp R, Knerr I, Dotsch J. Course of placental 11beta-hydroxysteroid dehydrogenase type 2 and 15-hydroxyprostaglandin dehydrogenase mRNA expression during human gestation. Eur J Endocrinol. 2001;145(2):187–192. doi: 10.1530/eje.0.1450187. [DOI] [PubMed] [Google Scholar]
- 86.Selye H. The stress of life. New York, NY: McGraw-Hill; 1956. [Google Scholar]
- 87.Serpeloni F, Radtke KM, Hecker T, Sill J, Vukojevic V, de Assis SG, Natt D. Does prenatal stress shape postnatal resilience? - an epigenome-wide study on violence and mental health in humans. Front Genet. 2019;10:269. doi: 10.3389/fgene.2019.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shanthikumar S, Neeland MR, Maksimovic J, Ranganathan SC, Saffery R. DNA methylation biomarkers of future health outcomes in children. Mol Cell Pediatr. 2020;7(1):7. doi: 10.1186/s40348-020-00099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sharma R, Frasch MG, Zelgert C, Zimmermann P, Fabre B, Wilson R, Antonelli MC. Maternal-fetal stress and DNA methylation signatures in neonatal saliva: an epigenome-wide association study. Clin Epigenetics. 2022;14(1):87. doi: 10.1186/s13148-022-01310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sharp GC, Arathimos R, Reese SE, Page CM, Felix J, Kupers LK, Zuccolo L. Maternal alcohol consumption and offspring DNA methylation: findings from six general population-based birth cohorts. Epigenomics. 2018;10(1):27–42. doi: 10.2217/epi-2017-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharp GC, Salas LA, Monnereau C, Allard C, Yousefi P, Everson TM, Relton CL. Maternal BMI at the start of pregnancy and offspring epigenome-wide DNA methylation: findings from the pregnancy and childhood epigenetics (PACE) consortium. Hum Mol Genet. 2017;26(20):4067–4085. doi: 10.1093/hmg/ddx290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shoemaker R, Deng J, Wang W, Zhang K. Allele-specific methylation is prevalent and is contributed by CpG-SNPs in the human genome. Genome Res. 2010;20(7):883–889. doi: 10.1101/gr.104695.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sosnowski DW, Booth C, York TP, Amstadter AB, Kliewer W. Maternal prenatal stress and infant DNA methylation: a systematic review. Dev Psychobiol. 2018;60(2):127–139. doi: 10.1002/dev.21604. [DOI] [PubMed] [Google Scholar]
- 94.Suarez A, Lahti J, Czamara D, Lahti-Pulkkinen M, Knight AK, Girchenko P, Raikkonen K. The epigenetic clock at birth: associations with maternal antenatal depression and child psychiatric problems. J Am Acad Child Adolesc Psychiatry. 2018;57(5):321–328. doi: 10.1016/j.jaac.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sutherland S, Brunwasser SM. Sex differences in vulnerability to prenatal stress: a review of the recent literature. Curr Psychiatry Rep. 2018;20(11):102. doi: 10.1007/s11920-018-0961-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Szyf M. The epigenetics of perinatal stress dialogues. Clin Neurosci. 2019;21(4):369–378. doi: 10.31887/DCNS.2019.21.4/mszyf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Teh AL, Pan H, Chen L, Ong ML, Dogra S, Wong J, Holbrook JD. The effect of genotype and in utero environment on interindividual variation in neonate DNA methylomes. Genome Res. 2014;24(7):1064–1074. doi: 10.1101/gr.171439.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tobi EW, Slieker RC, Stein AD, Suchiman HE, Slagboom PE, van Zwet EW, Lumey LH. Early gestation as the critical time-window for changes in the prenatal environment to affect the adult human blood methylome. Int J Epidemiol. 2015;44(4):1211–1223. doi: 10.1093/ije/dyv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Turecki G, Meaney MJ. Effects of the social environment and stress on glucocorticoid receptor gene methylation: a systematic review. Biol Psychiatry. 2016;79(2):87–96. doi: 10.1016/j.biopsych.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Urday P, Gayen NeeBetal S, Sequeira Gomes R, Al-Kouatly HB, Solarin K, Chan JS, Aghai ZH. SARS-CoV-2 Covid-19 infection during pregnancy and differential DNA methylation in human cord blood cells from term neonates. Epigenet Insights. 2023;16:25168657231184665. doi: 10.1177/25168657231184665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ursini G, Punzi G, Langworthy BW, Chen Q, Xia K, Cornea EA, Weinberger DR. Placental genomic risk scores and early neurodevelopmental outcomes. Proc Natl Acad Sci U S A. 2021 doi: 10.1073/pnas.2019789118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, Schwab M. Prenatal developmental origins of behavior and mental health: the influence of maternal stress in pregnancy. Neurosci Biobehav Rev. 2020;117:26–64. doi: 10.1016/j.neubiorev.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 103.Varley KE, Gertz J, Bowling KM, Parker SL, Reddy TE, Pauli-Behn F, Myers RM. Dynamic DNA methylation across diverse human cell lines and tissues. Genome Res. 2013;23(3):555–567. doi: 10.1101/gr.147942.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 105.Xiao FH, Wang HT, Kong QP. Dynamic DNA methylation during aging: a "prophet" of age-related outcomes. Front Genet. 2019;10:107. doi: 10.3389/fgene.2019.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yehuda R, Daskalakis NP, Bierer LM, Bader HN, Klengel T, Holsboer F, Binder EB. Holocaust exposure induced intergenerational effects on FKBP5 methylation. Biol Psychiatry. 2016;80(5):372–380. doi: 10.1016/j.biopsych.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 107.Zannas AS, Binder EB. Gene-environment interactions at the FKBP5 locus: sensitive periods, mechanisms and pleiotropism. Genes Brain Behav. 2014;13(1):25–37. doi: 10.1111/gbb.12104. [DOI] [PubMed] [Google Scholar]
- 108.Zhang W, Ham J, Li Q, Deyssenroth MA, Lambertini L, Huang Y, Nomura Y. Moderate prenatal stress may buffer the impact of superstorm sandy on placental genes: stress in pregnancy (SIP) study. PLoS ONE. 2020;15(1):e0226605. doi: 10.1371/journal.pone.0226605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.