Abstract
Symptoms common among individuals with multiple sclerosis (MS) may influence health promotion and quality of life, especially among older adults, who often experience multiple chronic conditions. To identify and examine symptom clusters’ effect on health promotion and quality of life, data from 215 adults with MS older than 60 (average years with diagnosis = 29) were analyzed. Correlations among symptoms ranged from 0.33 to 0.81. Factor analysis identified two symptom clusters: (a) physical/psychological/cognitive symptoms and (b) pain symptoms. In multiple hierarchical regressions, controlling for demographics and functional limitations, physical/psychological/cognitive symptoms significantly improved prediction on Health-Promoting Lifestyle Profile II inter-personal relations, stress management, and total scores; pain symptoms predicted nutrition scores. Both symptom clusters predicted spiritual growth and quality of life. Social support was a significant predictor of all outcomes. Symptom clusters, along with social support, should be considered in care and interventions for older adults with MS.
Multiple sclerosis (MS), a chronic disabling disease of the central nervous system that is thought to be immune-mediated, affects more than 2.3 million individuals worldwide (National Multiple Sclerosis Society, 2016). Most individuals with MS are diagnosed in their midlife, and it pre-dominantly afflicts women. With the development of medical therapies, the lifespan of individuals with MS has increased dramatically: approximately 90% of those with MS now in their 20s may live into their 70s, and currently, approximately one quarter of those with MS are older than 65 (Buhse, 2015). However, in a recent study (Marrie et al., 2015), median survival in the MS population was shorter than expected (75.9 years versus 83.4 years for matched controls). Older adults with MS tend to live with other chronic disabling comorbidities and poor quality of life (QoL) (Buhse, 2015; Marrie et al., 2015). Common symptoms related to MS include fatigue, pain, depression, stress, sleep disorders, and cognitive problems, which can co-occur to create functional barriers that influence an individual’s health promotion and QoL (Forbes, While, Mathes, & Griffiths, 2006; Kayes et al., 2011; Motl, Weikert, Suh, & Dlugonski, 2010; Newland, Fearing, Riley, & Neath, 2012; Nsamenang, Hirsch, Topciu, Goodman, & Duberstein, 2016; Shahrbanian, Duquette, Kuspinar, & Mayo, 2015; Williams, Vietri, Isherwood, & Flor, 2014).

According to Stuifbergen’s model (Stuifbergen, Seraphine, & Roberts, 2000), patients’ with MS health promotion behaviors and QoL are influenced by antecedent factors, which include demographics and functional limitations that result from MS, as well as factors that operate as barriers and facilitators related to health promotion (e.g., social support as a personal resource) (Figure). Although the initial model did not address the effect of symptoms, MS symptoms and symptom clusters can be conceptualized as barriers to health promotion and QoL. MS symptoms have been found to differ by disease duration (Vitkova et al., 2014). Older adults with MS tend to have longstanding MS (>17 years), but may have better symptom coping, health promotion behaviors, and QoL simply because they have learned how to manage their disease over time. However, it is also true that less physical activity has been reported in older adults with MS, which can impair management of their chronic condition’s progression and consequences (Klaren et al., 2016). Thus, how physical and psychological symptoms co-occur or cluster among older adults with longstanding MS and how such clusters might influence their health promotion behaviors and QoL merit further exploration, especially now that the number of those aging with MS is increasing. The purpose of the current study was to test contributions of symptom information with the prediction of key health promotion outcomes and QoL among older adults with MS. The following research questions were considered:
Figure.
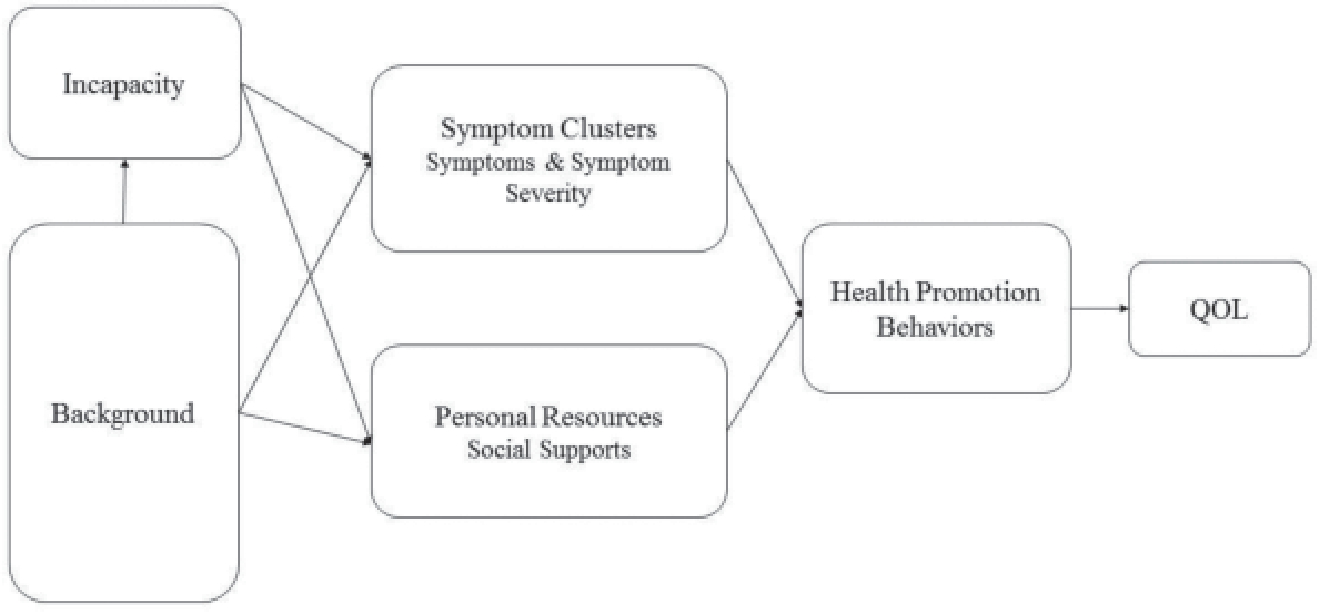
Contribution of symptom clusters to the model of health promotion and quality of life (QOL) for older adults with multiple sclerosis.
How do symptoms cluster among older adults with long-standing MS?
How do symptom clusters and personal resources (e.g., social support) contribute to the prediction of health promotion among older adults with longstanding MS after controlling for certain demographic variables and functional limitations?
How do symptom clusters, personal resources, and health promotion behaviors predict the QoL of older adults with longstanding MS after controlling for certain demographic variables and functional limitations?
METHOD
Data Collection
Data for these analyses were obtained in the 18th year of data collection for an ongoing longitudinal study of health promotion and QoL in individuals with MS (Stuifbergen, Blozis, Becker, Harrison, & Kullberg, 2016). Following institutional review board approval from the current authors’ university, the sample was originally recruited from a large mailing to the members of two National Multiple Sclerosis Society chapters in Texas. Some participants have since moved to other states, so the current sample is more diverse in location, but most still live in Texas. Recruitment of the initial sample has been described elsewhere (Stuifbergen & Roberts, 1997).
An introductory letter, survey, and postage-paid envelope were mailed to 396 participants in the spring of Year 18. In an effort to obtain complete data after surveys were returned, respondents who left items unanswered were mailed an additional letter that included copies of pages with missing data, which they were asked to provide. Respondents were told they could draw a line through any items they preferred not to answer. Data from 301 participants were received and usable, resulting in a 76% response rate. A total of 215 participants 60 or older were included in the current study.
Instruments
Background Information Sheet.
In previous surveys of this sample, a background information sheet was used to collect data for age, gender, ethnicity, education, marital status, employment status, and years since diagnosis. However, to reduce participants’ response burden, the Year 18 study collected information only on marital and employment status; other data were extrapolated from prior datasets.
Incapacity Status Scale.
Functional impairments due to MS were evaluated with the Incapacity Status Scale (ISS; Kurtzke, 1981). The ISS includes 16 items rated on a 5-point scale, with higher scores indicating greater inability to perform activities and total scores ranging from 0 to 64. The scale’s validity has been supported by Kurtzke (1981) and Stuifbergen, Blozis, Harrison, and Becker (2006). Cronbach’s alpha for the ISS in the current study was 0.87.
Personal Resource Questionnaire.
Social support was measured with the Personal Resource Questionnaire (PRQ), the validity of which has been supported with various samples (Weinert & Brandt, 1987). The PRQ’s 25 items are scaled from 1 = strongly disagree to 7 = strongly agree; total scores range from 25 to 175. Higher scores indicate higher perceived social support. Cronbach’s alpha for the PRQ in the current study was 0.94.
Perceived Stress Scale.
Symptoms of perceived stress were measured with the Perceived Stress Scale (PSS; Cohen, Kamarck, & Mermelstein, 1983), the validity of which has been supported in individuals with MS (Strober & Arnett, 2016). The PSS has 10 items, each scaled from 0 = never to 4 = very often; total scores range from 0 to 40. Cronbach’s alpha for the PSS in the current study was 0.89.
The Center for Epidemiologic Studies Depression Scale-10.
Depressive symptoms were measured with the Center for Epidemiologic Studies Depression Scale-10 (Radloff, 1977), which has been shown to be a reliable and valid measure in various populations (Andresen, Malmgren, Carter, & Patrick, 1994; Stuifbergen et al., 2000). Responses on the 10 items, scaled from 0 to 3, represent how frequently patients have experienced 10 depressive symptoms in the past week. For the current study, Cronbach’s alpha was 0.87.
Patient Reported Outcomes Measurement Information System Items for Pain, Fatigue, Sleep, and Cognitive Abilities.
The Patient Reported Outcomes Measurement Information System (PROMIS) is “a set of person-centered measures that evaluates and monitors physical, social, and emotional health” (Northwestern University, 2017, para. 1). PROMIS version 1.0 short forms for Pain Interference (6 items), Pain Intensity (3 items), Fatigue (7 items), Sleep Disturbance (7 items), and Applied Cognition Abilities (6 items) were used to evaluate these symptoms. Higher scores represent more of the construct being measured. The PROMIS scales’ reliability and validity have been tested in various populations (Cella et al., 2007). For the PROMIS scales used in the current study, Cronbach’s alphas ranged from 0.84 to 0.95.
Health-Promoting Lifestyle Profile II.
The frequency of health promotion activities was measured by the Health-Promoting Lifestyle Profile II (HPLP II). This measure includes 52 items organized into six subscales (i.e., physical activity, health responsibility, spirtual growth, interpersonal relations, nutrition, and stress management) (Walker, Sechrist, & Pender, 1995). Respondents are asked how often they perform each activity on a scale from 1 to 4 (where 1 = never, 2 = sometimes, 3 = often, or 4 = routinely). The scale’s reliability and validity have been supported in previous studies of individuals with MS (Stuifbergen, Becker, Blozis, Timmerman, & Kullberg, 2003). For the current study, Cronbach’s alpha ranged from 0.79 to 0.89 and was 0.94 for the total score.
Self-Rated Quality of Life.
The question “How would you rate your overall quality of life at this time?” provided self-rated QoL scores rated from 1 = very poor to 10 = very good. Minh, Ng, Byass, and Wall’s (2012) study supports the validity of a similar 1-item global rating of QoL for older adults.
Data Analysis
Surveys were proofed for completeness and entered into SPSS version 23. Data were checked for out-of-range values. In addition, data entry was double checked for a random sample of 10% of the sample; error rates were <1%. For the current study, <15% of data were missing for each scale. Therefore, mean substitution was used to account for missing data.
Marital status was recoded into two groups: married/significant others and unmarried. Employment status was recoded into three groups: employed, unemployed not due to disability, and unemployed due to disability.
Because of the risk of multicolinearity when predictors are highly related, factor scores were derived to cluster the examined symptoms. Consistent with the procedure used by Shahrbanian et al. (2015) to identify MS symptom clusters, principal component factor analysis was used to compute symptom cluster factor scores for each participant. Because the scales contained different numbers of items, the Z scores of the symptom scales were used so that the symptom measures were equally weighted in the composite score. Multiple hierarchical regression was used to predict health promotion and QoL outcomes. Consistent with the model shown in the Figure, demographic variables, including age, years of education, and years since diagnosis, and functional limitations (ISS scores) were input in the first step of the regression. Factor analysis scores representing the symptom clusters and social support (PRQ scores) were input in the second step. For the hierarchical regression for QoL, the HPLP II total score was added in the third step. Alpha was set at p < 0.05 for all analyses.
RESULTS
Sample Description
Participants’ ages ranged from 60 to 90 years (mean = 68 years, SD = 6.61 years) (Table 1). Mean time since diagnosis was 28.67 years (SD = 6.96 years), and 37.6% were taking disease-modifying medications. Most of the sample was married (65.1%), non-Hispanic White individuals (95.8%), and female (87%). Most (87%) had a high school education or higher. Few (9.3%) had benign sensory MS, 38.1% had relapsing-remitting MS, and 40.4% had progressive MS. Only 10.7% (n = 23) were employed (full- or part-time), and 19.5% (n = 42) reported being unemployed due to disability. Mean QoL rating (where 1 = very poor and 10 = very good) was 7.45 (SD = 2.2), and 46.1% rated their health as fair or poor.
TABLE 1.
SAMPLE BACKGROUND CHARACTERISTICS (N = 215)
| Variable | n (%) |
|---|---|
| Gender | |
| Female | 187 (87) |
| Male | 28 (13) |
| Age (years) | |
| <65 | 70 (32.6) |
| 65 to 85 | 143 (66.5) |
| >85 | 2 (0.9) |
| Ethnicity | |
| Non-Hispanic White | 206 (95.8) |
| Other | 9 (4.2) |
| Education | |
| <High school | 28 (13) |
| High school | 67 (31.2) |
| Associate's degree | 25 (11.6) |
| Bachelor's degree | 61 (28.4) |
| Graduate degree | 34 (15.8) |
| Marital status | |
| Married | 140 (65.1) |
| Unmarried | 75 (34.9) |
| Employment status | |
| Retired | 122 (56.7) |
| Unemployed due to disability | 42 (19.5) |
| Homemaker | 22 (10.3) |
| Full-time | 14 (6.5) |
| Part-time | 9 (4.2) |
| Unemployed due to age | 5 (2.3) |
| Fired/laid off | 1 (0.5) |
| Multiple sclerosis typea | |
| Benign sensory | 20 (9.3) |
| Relapsing remitting | 82 (38.1) |
| Primary progressive | 36 (16.7) |
| Secondary progressive | 45 (20.9) |
| Progressive relapsing | 6 (2.8) |
| Unable to say | 24 (11.2) |
| Taking disease-modifying prescription | |
| No | 134 (62.3) |
| Yes | 81 (37.7) |
| Mean (SD) | |
| Age (years) | 68.44 (6.61) |
| Years since diagnosis | 28.67 (6.96) |
Two participants did not answer this question.
Symptom Clusters
Table 2 shows the correlations between individual symptoms. Correlation values ranged from 0.33 to 0.81, indicating many were highly and significantly (p < 0.001) related, co-occurring as clusters.
TABLE 2.
SUMMARY OF CORRELATIONS, MEANS, AND STANDARD DEVIATIONS OF SYMPTOM MEASURES (N = 215)a
| Symptom | Mean (SD) | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| 1. CES-D 10b | 8.79 (6.23) | ||||||
| 2. Stress | 14.32 (7.9) | 0.76 | |||||
| 3. Pain interference | 14.64 (7.1) | 0.44 | 0.44 | ||||
| 4. Pain intensity | 7.28 (2.97) | 0.33 | 0.33 | 0.81 | |||
| 5. Fatigue | 20.41 (6.87) | 0.60 | 0.55 | 0.61 | 0.48 | ||
| 6. Sleep | 20.58 (7.8) | 0.50 | 0.48 | 0.44 | 0.40 | 0.42 | |
| 7. Cognitive ability | 21.03 (6.3) | −0.55 | −0.54 | −0.40 | −0.33 | −0.63 | −0.41 |
Note. CES-D 10 = Center for Epidemiologic Studies Depression Scale-10.
All p < 0.01.
N = 214.
Based on the scree plot of the factor analysis, two factor clusters were derived from the principal components factor analysis. Fatigue, depression, stress, sleep, and cognitive ability comprised the first cluster: physical/psychological/cognitive symptoms. The pain intensity and pain interference scales loaded together to constitute the second factor: pain symptoms.
Multiple Regression Analyses
Multiple hierarchical regressions were used to examine the contributions of symptom cluster scores and perceived social support to the prediction of HPLP II scores and QoL measure after controlling for demographic and functional limitations. Assumptions underlying multivariate analysis were met. The results showed that scores for the two symptom factor clusters and social support (PRQ score) explained significant variability in the HPLP II total and many of its subscales among older adults with MS after controlling for selected demographic variables and incapacity status (Table 3). Background characteristics entered on the first step contained significant predictors in many models. At the second step, the physical/psychological/cognitive symptom cluster added significantly to the prediction of HPLP II inter-personal relations, stress management, spiritual growth, and HPLP II total. The pain cluster was a significant predictor for the HPLP II nutrition and spiritual growth subscales. Interestingly, the pain cluster was positively correlated with scores on the spiritual subscale. The social support score was a significant predictor in all models. R2 for the final models ranged from 0.16 to 0.6. The HPLP II total score, both symptom cluster scores, and social support score also added significantly to the prediction of QoL after controlling for certain demographic variables and incapacity status. R2 for the final model to predict QoL was 0.51.
TABLE 3.
SUMMARY OF MULTIPLE HIERARCHICAL REGRESSION RESULTS
| Model Estimate | B | β | t | p Value |
|---|---|---|---|---|
| HPLP II health responsibility subscale (N = 214)a | ||||
| Constant | 10.19 | — | 1.88 | 0.06 |
| Age (years) | 0.02 | 0.02 | 0.28 | 0.78 |
| Years since diagnosis | −0.01 | −0.01 | −0.20 | 0.85 |
| Years of education | 0.15 | 0.07 | 1.03 | 0.30 |
| Multiple sclerosis incapacity (ISS) | 0.05 | 0.09 | 1.26 | 0.21 |
| Physical/psychological/cognitive cluster | −0.82 | −0.15 | −1.52 | 0.13 |
| Pain cluster | 0.28 | 0.05 | 0.69 | 0.49 |
| Social support (PRQ) | 0.07 | 0.31 | 3.81 | <0.001*** |
| HPLP II physical activity subscale (N = 214)b | ||||
| Constant | 11.63 | — | 2.18 | 0.03* |
| Age (years) | −0.03 | −0.03 | −0.45 | 0.65 |
| Years since diagnosis | 0.03 | 0.03 | 0.57 | 0.57 |
| Years of education | 0.32 | 0.13 | 2.25 | 0.03* |
| Multiple sclerosis incapacity (ISS) | −0.30 | −0.47 | −7.08 | <0.001*** |
| Physical/psychological/cognitive cluster | 0.24 | 0.04 | 0.45 | 0.66 |
| Pain cluster | −0.52 | −0.09 | −1.30 | 0.20 |
| Social support (PRQ) | 0.04 | 0.17 | 2.43 | 0.02* |
| HPLP II nutrition subscale (N = 213)c | ||||
| Constant | 3.27 | — | 0.65 | 0.52 |
| Age (years) | 0.11 | 0.13 | 2.04 | 0.04* |
| Years since diagnosis | 0.01 | 0.01 | 0.22 | 0.83 |
| Years of education | 0.38 | 0.17 | 2.79 | 0.01* |
| Multiple sclerosis incapacity (ISS) | −0.05 | −0.09 | −1.23 | 0.22 |
| Physical/psychological/cognitive cluster | −0.40 | −0.07 | −0.80 | 0.43 |
| Pain cluster | −0.88 | −0.16 | −2.33 | 0.02* |
| Social support (PRQ) | 0.07 | 0.30 | 4.00 | <0.001*** |
| HPLP II spiritual subscale (N = 214)d | ||||
| Constant | 7.16 | — | 1.73 | 0.09 |
| Age (years) | 0.07 | 0.08 | 1.64 | 0.10 |
| Years since diagnosis | −0.04 | −0.05 | −1.06 | 0.29 |
| Years of education | −0.04 | −0.02 | −0.32 | 0.75 |
| Multiple sclerosis incapacity (ISS) | 0.02 | 0.04 | 0.64 | 0.53 |
| Physical/psychological/cognitive cluster | −1.85 | −0.33 | −4.51 | <0.001*** |
| Pain cluster | 0.65 | 0.11 | 2.09 | 0.04* |
| Social support (PRQ) | 0.13 | 0.53 | 8.88 | <0.001*** |
| HPLP II interpersonal relations subscale (N = 214)e | ||||
| Constant | 2.28 | — | 0.68 | 0.50 |
| Age (years) | 0.06 | 0.08 | 1.77 | 0.08 |
| Years since diagnosis | 0.02 | 0.02 | 0.51 | 0.61 |
| Years of education | 0.11 | 0.06 | 1.24 | 0.22 |
| Multiple sclerosis incapacity (ISS) | 0.02 | 0.03 | 0.60 | 0.55 |
| Physical/psychological/cognitive cluster | −0.74 | −0.15 | −2.25 | 0.03* |
| Pain cluster | 0.33 | 0.07 | 1.30 | 0.20 |
| Social support (PRQ) | 0.14 | 0.69 | 12.28 | <0.001*** |
| HPLP II stress management subscale (N = 213)f | ||||
| Constant | 9.57 | — | 2.47 | 0.01* |
| Age (years) | 0.06 | 0.08 | 1.39 | 0.17 |
| Years since diagnosis | 0.07 | 0.10 | 1.71 | 0.09 |
| Years of education | 0.05 | 0.03 | 0.44 | 0.66 |
| Multiple sclerosis incapacity (ISS) | −0.04 | −0.07 | −1.14 | 0.25 |
| Physical/psychological/cognitive cluster | −1.31 | −0.29 | −3.42 | <0.001*** |
| Pain cluster | −0.20 | −0.04 | −0.69 | 0.49 |
| Social support (PRQ) | 0.06 | 0.31 | 4.47 | <0.001*** |
| HPLP II total (N = 212)g | ||||
| Constant | 42.75 | — | 2.41 | 0.02* |
| Age (years) | 0.29 | 0.08 | 1.56 | 0.12 |
| Years since diagnosis | 0.08 | 0.02 | 0.44 | 0.66 |
| Years of education | 1.04 | 0.11 | 2.17 | 0.03* |
| Multiple sclerosis incapacity (ISS) | −0.31 | −0.12 | −2.19 | 0.03* |
| Physical/psychological/cognitive cluster | −4.80 | −0.20 | −2.74 | 0.01* |
| Pain cluster | −0.37 | −0.02 | −0.28 | 0.78 |
| Social support (PRQ) | 0.51 | 0.51 | 8.38 | <0.001*** |
| Quality of life (N = 212)h | ||||
| Constant | 4.84 | — | 2.85 | <0.01** |
| Age (years) | −0.03 | −0.07 | −1.39 | 0.17 |
| Years since diagnosis | 0 | −0.01 | −0.21 | 0.83 |
| Years of education | −0.04 | −0.05 | −0.88 | 0.38 |
| Multiple sclerosis incapacity (ISS) | −0.04 | −0.19 | −3.26 | <0.01** |
| Physical/psychological/cognitive cluster | −0.36 | −0.16 | −2.14 | 0.03* |
| Pain cluster | −0.40 | −0.18 | −3.15 | <0.01** |
| Social support (PRQ) | 0.02 | 0.19 | 2.64 | <0.01** |
| HPLP II total | 0.02 | 0.25 | 3.40 | <0.001*** |
Note. HPLPII = Health-Promoting Lifestyle Profile II; ISS = Incapacity Status Scale; PRQ = Personal Resource Questionnaire.
p < 0.05;
p < 0.01;
p < 0.001.
Model 1: R2 = 0.02, F = 1.30; Model 2: R2 = 0.16, ΔF = 10.61***, F = 5.39***.
Model 1: R2 = 0.31, F = 23.15***; Model 2: R2 = 0.34, ΔF = 3.07*, F = 14.93***.
Model 1: R2 = 0.14, F = 8.66***; Model 2: R2 = 0.28, ΔF = 13.37***, F = 11.56***.
Model 1: R2 = 0.09, F = 5.34***; Model 2: R2 = 0.54, ΔF = 65.28***, F = 33.84***.
Model 1: R2 = 0.10, F = 5.71***; Model 2: R2 = 0.60, ΔF = 85.45***, F = 43.83***.
Model 1: R2 = 0.14, F = 8.73***; Model 2: R2 = 0.38, ΔF = 25.41***, F = 17.63***.
Model 1: R2 = 0.20, F = 13.16***; Model 2: R2 = 0.54, ΔF = 49.92 ***, F = 34.24***
Model 1: R2 = 0.23, F = 15.69***; Model 2: R2 = 0.48, ΔF = 31.72***, F = 26.55***; Model 3: R2 = 0.51, ΔF = 11.53 ***, F = 25.87***.
DISCUSSION
The current study provides evidence for symptom clusters and how they influence health promotion behaviors and QoL in older adults with longstanding MS. As expected, the examined symptoms (i.e., depression, fatigue, stress, pain intensity, pain interference, sleep disturbance, and cognitive ability) were highly related. Principal components factor analysis yielded two symptom clusters as factors: physical/psychological/cognitive symptoms and pain symptoms. These symptom clusters, together with social supports, added significantly to the prediction of the HPLP II total score and scores on four of its subscales after controlling for demographics and functional limitations associated with MS. By contrast, only social support was a significant predictor for HPLP II health responsibility, and only functional limitations, years of education, and social support were significant predictors for HPLP II physical activity. Although accounted for variance ranged from 0.16 to 0.6, all equations were statistically significant at p < 0.001. Accounted for variance on the health responsibility subscale was lower than that for the other scales, and to better predict it, future studies should include more potentially related variables such as health literacy. Interestingly, the pain cluster score was positively related to the score on the spiritual subscale of the HPLP II, suggesting individuals in pain may seek spiritual support to help cope with their pain.
With respect to the third research question (i.e., How do symptom clusters, social support, and health promotion behaviors predict the QoL of older adults with long-standing MS?), both symptom cluster factor scores, along with the social support score, added significantly to the prediction of QoL after controlling for selected background characteristics (including functional limitations). HPLP II total scores added significant unique variance to the prediction of QoL on the third step of this equation. This finding suggests that engaging in health promotion can contribute to QoL for older adults with MS beyond the effects of demographic and disease-related characteristics.
Although some of the current study’s findings are similar to those of other studies, there are some differences that may be attributable to different variables or different measures used across studies. For example, most previous studies have examined bivariate relationships between symptoms and various outcomes, whereas the current study used hierarchical regression to control for the contribution of demographics and functional limitations before examining the relationships among symptom clusters, social support, health promotion, and QoL. Compared with the sample in Shahrbanian et al.’s (2015) study on the contribution of symptom clusters to MS consequences, the sample in the current study was older and more highly educated, with more progressive longstanding MS—characteristics that might have contributed to the complex relationships of the symptoms and different findings for symptom clusters. Previous studies have reported symptom clusters in different ways (Motl et al., 2010; Newland et al., 2012; Nsamenang et al., 2016; Shahrbanian, Duquette, Ahmed, & Mayo, 2016; Williams et al., 2014), but the current study found that sleep, fatigue, stress, depression, and cognitive abilities constituted one cluster, whereas the two pain scales constituted a separate cluster. The importance of pain clustering separately from the other symptoms may be due to the significance of pain associated with comorbid conditions experienced by older adults with MS.
Unlike in Motl et al.’s (2010) study, after controlling for functional limitations and demoraphics, the current study found that neither symptom cluster significantly predicted the score on the HPLP II physical activity subscale. This finding indicates that compared to the younger population, MS incapacity and social support may play a greater role in influencing physical activity in older adults with MS. The findings on the relationships among the symptoms, spiritual growth, and QoL were consistent with those of prior studies (Nsamenang et al., 2016; Shahrbanian et al., 2015; Williams et al., 2014). Variances of the health promotion behaviors explained by the variables examined in the current study ranged from 16% to 60%, and together with HPLP II total, age, years since diagnosis, years of education, functional limitation, symptom clusters, and social support, they accounted for 51% of variance in QoL, highlighting the overall importance of this constellation of variables in individuals’ lives. Except for the model of HPLP II health responsibility, which explained a moderate variance, all other models explained large variances of the outcomes.
LIMITATIONS
The current study has the following limitations. First, a convenience sample of individuals originally recruited through the MS Society in Texas was used. Some of them had since moved to other states, and most were non-Hispanic White individuals. The results thus may not be generalizable to other older adults with MS. Future studies should use more diverse samples recruited with other sampling strategies. Second, the biases of self-report measures, such as social desirability and response set, exist because of the subjective nature of self-report data. Future studies should explore how symptom clusters change over time and how those changes are influenced by sociocultural factors, such as loneliness and marital status. In addition, participants were “survivors” in an 18-year longitudinal study who might therefore behave differently with respect to health promotion and have a different QoL experience than other groups of individuals with longstanding MS. Because the current study was a correlational analysis, causality cannot be determined. Finally, it should be remembered that given the large sample, small correlations might be statistically significant yet may not all correspond to meaningful impacts in the daily lives of individuals with MS.
IMPLICATIONS FOR NURSING PRACTICE
Older adults with MS may experience multiple symptoms, including pain, fatigue, depression, stress, decreasing cognitive abilities, and sleep disturbance. The co-occurrence of these symptoms can affect health-promoting abilities and QoL beyond the functional limitations imposed by MS itself. Nurses should consider the co-occurrence of symptoms in conducting their nursing assessment in addition to the functional limitations associated with MS so that they can provide more comprehensive care and effective interventions (Buhse, 2015). Patients who are challenged to manage multiple symptoms within the context of a debilitating chronic condition may find it important to have an empathetic health care provider who can offer guidance that considers the big picture of what they are dealing with.
Consistent with previous research, the current study also underscores the powerful impact of social support on health promotion and QoL for older individuals with MS. Nurses should therefore seek ways to enhance the social supports available to older adults with MS to promote their health and QoL. For example, nurses should provide time for older adults with MS to discuss their experience and feelings; encourage the use of follow-up phone calls, support groups, or community activities; and ensure the proper use of mobility equipment to help avoid social isolation (Buhse, 2015). Nurses can educate patients about previous research, demonstrating the positive impact of group interventions to promote health promotion and QoL for individuals with MS (Calandri, Graziano, Borghi, & Bonino, 2016; Kos et al., 2016; Stuifbergen et al., 2003). Nurses should be knowledgeable about resources available from their state’s Office for the Aging, the National MS Society, and other organizations so that they can share this information with patients. In addition, the impact of comorbidity should be considered in clinical practice. Consistent with recommendations for patient-centered care of patients with multiple chronic conditions, nurses should be prepared to refer patients with MS for general screening and treatment of comorbid conditions that may impact their ability to promote their health (American Geriatrics Society Expert Panel on Person-Centered Care, 2016; Buhse, 2015).
CONCLUSION
The current findings suggest that symptoms experienced by older adults with longstanding MS tend to cluster or co-occur, and that clusters of symptoms are associated with certain health promotion behaviors and QoL. Perceived social support was the strongest predictor of health promoting outcomes in all areas but physical activity. To provide comprehensive care and interventions, health providers who care for individuals with MS and researchers should consider the impacts of co-occurring symptoms, functional impairments, and limited social support among patients in clinical practices when deciding how to promote health and improve QoL. Future studies and interventions should focus on coping strategies that improve symptom management and social support to promote health and QoL.
Acknowledgments
The authors acknowledge Vicki K. Kullberg for project management, and Ashley Henneghan, Lauren Culp, and Nicki Gloris for data entry and checking.
Footnotes
The authors have disclosed no potential conflicts of interest, financial or otherwise. This study was supported in part with grant funding from the National Institutes of Health and National Institute of Nursing Research (Dr. Stuifbergen; R01 NR003195) and by the James R. Dougherty Jr. Centennial Professorship in Nursing at the University of Texas at Austin. Editorial support was provided by the Cain Center for Nursing Research and the Center for Transdisciplinary Collaborative Research in Self-Management Science (P30, NR015335) at the University of Texas at Austin School of Nursing.
Contributor Information
Wenhui Zhang, School of Nursing, University of Texas at Austin, Austin, Texas..
Heather Becker, School of Nursing, University of Texas at Austin, Austin, Texas..
Alexa K. Stuifbergen, School of Nursing, University of Texas at Austin, Austin, Texas..
Adama Brown, School of Nursing, University of Texas at Austin, Austin, Texas..
REFERENCES
- American Geriatrics Society Expert Panel on Person-Centered Care. (2016). Person-centered care: A definition and essential elements. Journal of the American Geriatrics Society, 64, 15–18. doi: 10.1111/jgs.13866 [DOI] [PubMed] [Google Scholar]
- Andresen EM, Malmgren JA, Carter WB, & Patrick DL (1994). Screening for depression in well older adults: Evalation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). American Journal of Preventive Medicine, 10, 77–84. [PubMed] [Google Scholar]
- Buhse M (2015). The elderly person with multiple sclerosis: Clinical implications for the increasing life-span. Journal of Neuroscience Nursing, 47, 333–339. doi: 10.1097/JNN.0000000000000172 [DOI] [PubMed] [Google Scholar]
- Calandri E, Graziano F, Borghi M, & Bonino S (2016). Improving the quality of life and psychological well-being of recently diagnosed multiple sclerosis patients: Preliminary evaluation of a group-based cognitive behavioral intervention. Disability and Rehabilitation. Advance online publication. doi: 10.1080/09638288.2016.1198430 [DOI] [PubMed] [Google Scholar]
- Cella D, Yount S, Rothrock N, Gershon R, Cook B, Reeve B,...Rose M (2007). The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH-Roadmap cooperative group during its first two years. Medical Care, 45(Suppl. 1), S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. [PubMed] [Google Scholar]
- Forbes A, While A, Mathes L, & Griffiths P (2006). Health problems and health-related quality of life in people with multiple sclerosis. Clinical Rehabilitation, 20, 67–78. doi: 10.1191/0269215506cr880oa [DOI] [PubMed] [Google Scholar]
- Kayes NM, McPherson KM, Schluter P, Taylor D, Leete M, & Kolt GS (2011). Exploring the facilitators and barriers to engagement in physical activity for people with multiple sclerosis. Disability and Rehabilitation, 33, 1043–1053. doi: 10.3109/09638288.2010.520801 [DOI] [PubMed] [Google Scholar]
- Klaren RE, Sebastiao E, Chiu C-Y, Kinnett-Hopkins D, McAuley E, & Motl RW (2016). Levels and rates of physical activity in older adults with multiple sclerosis. Aging and Disease, 7, 278–284. doi: 10.14336/AD.2015.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos D, Duportail M, Meirte J, Meeus M, D’hooghe MB, Nagels G,…Nijs J (2016). The effectiveness of a self-management occupational therapy intervention on activity performance in individuals with multiple sclerosis-related fatigue: A randomized-controlled trial. International Journal of Rehabilitation Research, 39, 255–262. doi: 10.1097/MRR.0000000000000178 [DOI] [PubMed] [Google Scholar]
- Kurtzke JF (1981). A proposal for a uniform minimal record of disability in multiple sclerosis. Acta Neurologica Scandinavica, 64, 110–129. doi: 10.1111/j.1600-0404.1981.tb05548.x [DOI] [Google Scholar]
- Marrie RA, Elliott L, Marriott J, Cossoy M, Blanchard J, Leung S, & Yu N (2015). Effect of comorbidity on mortality in multiple sclerosis. Neurology, 85, 240–247. doi: 10.1212/WNL.0000000000001718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minh HV, Ng N, Byass P, & Wall S (2012). Patterns of subjective quality of life among older adults in rural Vietnam and Indonesia. Geriatrics G Gerontology International, 12, 397–404. doi: 10.1111/j.1447-0594.2011.00777.x [DOI] [PubMed] [Google Scholar]
- Motl RW, Weikert M, Suh Y, & Dlugonski D (2010). Symptom cluster and physical activity in relapsing-remitting multiple sclerosis. Research in Nursing G Health, 33, 398–412. doi: 10.1002/nur.20396 [DOI] [PubMed] [Google Scholar]
- National Multiple Sclerosis Society. (2016). What is multiple sclerosis? Retrieved from http://www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Brochures/Brochure-What-Is-MS.pdf
- Newland PK, Fearing A, Riley M, & Neath A (2012). Symptom clusters in women with relapsing-remitting multiple sclerosis. Journal of Neuroscience Nursing, 44, 66–71. doi: 10.1097/JNN.0b013e3182478cba [DOI] [PubMed] [Google Scholar]
- Northwestern University. (2017). PROMIS. Retrieved from http://www.healthmeasures.net/explore-measurement-systems/promis
- Nsamenang SA, Hirsch JK, Topciu R, Goodman AD, & Duberstein PR (2016). The interrelations between spiritual well-being, pain interference and depressive symptoms in patients with multiple sclerosis. Journal of Behavioral Medicine, 39, 355–363. doi: 10.1007/s10865-016-9712-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Shahrbanian S, Duquette P, Ahmed S, & Mayo NE (2016). Pain acts through fatigue to affect participation in individuals with multiple sclerosis. Quality of Life Research, 25, 477–491. doi: 10.1007/s11136-015-1098-0 [DOI] [PubMed] [Google Scholar]
- Shahrbanian S, Duquette P, Kuspinar A, & Mayo NE (2015). Contribution of symptom clusters to multiple sclerosis consequences. Quality of Life Research, 24, 617–629. doi: 10.1007/s11136-014-0804-7 [DOI] [PubMed] [Google Scholar]
- Strober LB, & Arnett PA (2016). Unemployment among women with multiple sclerosis: The role of coping and perceived stress and support in the workplace. Psychology, Health G Medicine, 21, 496–504. doi: 10.1080/13548506.2015.1093645 [DOI] [PubMed] [Google Scholar]
- Stuifbergen AK, Becker H, Blozis S, Timmerman G, & Kullberg V (2003). A randomized clinical trial of a wellness intervention for women with multiple sclerosis. Archives of Physical Medicine and Rehabilitation, 84, 467–476. doi: 10.1053/apmr.2003.50028 [DOI] [PubMed] [Google Scholar]
- Stuifbergen AK, Blozis S, Becker H, Harrison T, & Kullberg V (2016). Selected health behaviors moderate the progression of functional limitations in persons with multiple sclerosis: Eleven years of annual follow-up. Disability and Health Journal, 9, 472–478. doi: 10.1016/j.dhjo.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuifbergen AK, Blozis SA, Harrison TC, & Becker HA (2006). Exercise, functional limitations, and quality of life: A longitudinal study of persons with multiple sclerosis. Archives of Physical Medicine and Rehabilitation, 87, 935–943. doi: 10.1016/j.apmr.2006.04.003 [DOI] [PubMed] [Google Scholar]
- Stuifbergen AK, & Roberts GJ (1997). Health promotion practices of women with multiple sclerosis. Archives of Physical Medicine and Rehabilitation, 78(Suppl. 5), S3–S9. doi: 10.1016/S0003-9993(97)90215-X [DOI] [PubMed] [Google Scholar]
- Stuifbergen AK, Seraphine A, & Roberts G (2000). An explanatory model of health promotion and quality of life in chronic disabling conditions. Nursing Research, 49, 122–129. [DOI] [PubMed] [Google Scholar]
- Vitkova M, Gdovinova Z, Rosenberger J, Szilasiova J, Nagyová I, Mikula P,… van Dijk JP (2014). Factors associated with poor sleep quality in patients with multiple sclerosis differ by disease duration. Disability and Health Journal, 7, 466–471. doi: 10.1016/j.dhjo.2014.05.004 [DOI] [PubMed] [Google Scholar]
- Walker SN, Sechrist KR, & Pender NJ (1995). Health-Promoting Lifestyle Profile II. Omaha, NE: University of Nebraska Medical Center. [Google Scholar]
- Weinert C, & Brandt PA (1987). Measuring social support with the Personal Resource Questionnaire. Western Journal of Nursing Research, 9, 589–602. doi: 10.1177/019394598700900411 [DOI] [PubMed] [Google Scholar]
- Williams AE, Vietri JT, Isherwood G, & Flor A (2014). Symptoms and association with health outcomes in relapsing-remitting multiple sclerosis: Results of a US patient survey. Multiple Sclerosis International, 2014, 1–8. doi: 10.1155/2014/203183 [DOI] [PMC free article] [PubMed] [Google Scholar]


