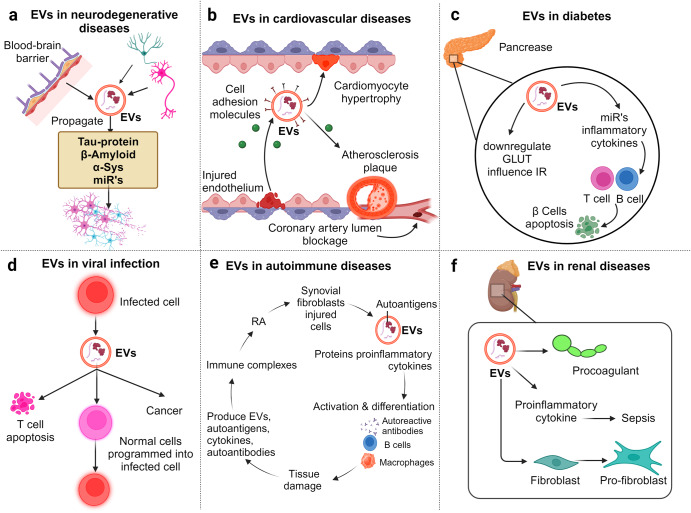
Fig. 6.
Pathological implications of extracellular vesicles in disease progression. a Neurological disorders. Cells such as microglia, astrocytes, and endothelial cells release EVs that transport neuropathological proteins like Aβ, Tau, and α-synuclein alongside specific miRNAs. These EVs can spread these harmful components across the brain, potentially accelerating neurodegenerative processes. Moreover, certain neurotoxic EVs can traverse the blood-brain barrier, disseminating their deleterious cargo to other neurons and amplifying neural dysfunction. b Cardiovascular Complications: EVs emanating from inflamed or damaged endothelial cells, as well as those from inflammatory cells, often display cell adhesion molecules and procoagulant markers. When the endothelium is compromised, these EVs adhere to the vascular wall, potentially instigating cardiomyocyte hypertrophy. Notably, patients suffering from acute coronary syndrome (ACS) demonstrate elevated concentrations of these circulating procoagulant endothelial microparticles. Over time, these contribute to atherosclerotic plaque formation, eventually risking blockage of coronary arteries. c Diabetes: In the diabetic milieu, EVs released within the pancreas carry detrimental miRNAs and pro-inflammatory cytokines. These EVs can instigate the apoptosis of insulin-producing β cells by activating specific immune cells, namely B and T cells. Furthermore, in type 2 diabetes (T2D), these EVs potentially reduce the efficacy of glucose transporters, exacerbating the disease. d Viral Infections & Oncogenesis: Cells infected by specific pathogens generate EVs that encapsulate viral components, facilitating their transfer to healthy cells. Some pathogens possess the capability to modify the content of EVs, leading to scenarios like T-cell apoptosis, potentially promoting oncogenesis. e Autoimmune Diseases: Physiological stress can spur the release of EVs enriched with autoantigens, inflammatory cytokines, and specific genetic elements (e.g., miRNA, DNA). These EVs can bind with antibodies and platelets to create immune complexes (ICs). These ICs then prime antigen-presenting cells, culminating in an intensified autoimmune reaction. This cascade can lead to heightened cellular destruction, as seen with the release of EVs from damaged synovial fibroblasts in RA. f Renal Disease & Organ Interplay: EVs circulating systemically facilitate communication between organs, and their dysregulated activities have been implicated in amplifying kidney damage and inflammation. These vesicles can potentially exacerbate renal ailments by fostering detrimental cellular interactions and inflammatory responses