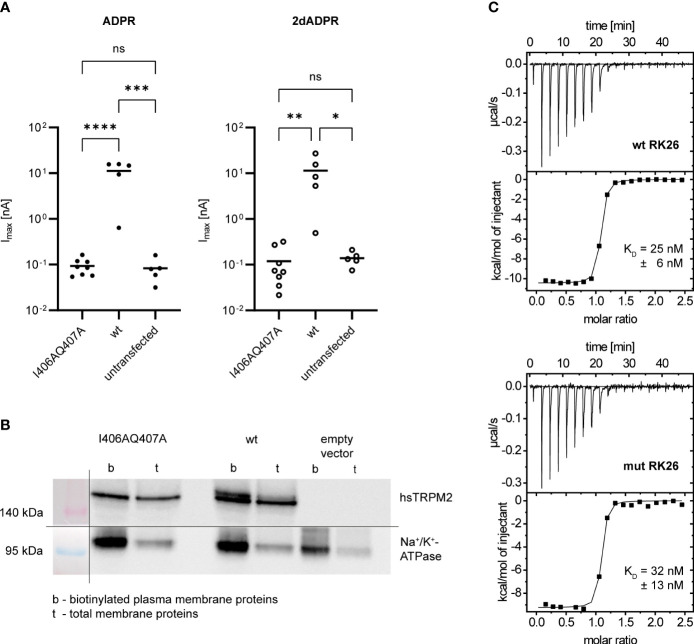
Figure 3.
(A) Whole-cell patch clamp experiments were performed 24 h after HEK293 cells were transiently transfected with an expression vector containing mutated (I406AQ407A) or wild type (wt) human TRPM2. For negative controls cells were left untransfected. The intracellular solution contained 10 µM Ca2+ and 100 µM ADPR/2dADPR. The extracellular solution contained 1 mM Ca2+. Data are presented on a log-scale. The bar denotes the mean. ns not significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 (one-way ANOVA). (B) Expression of human TRPM2 (hsTRPM2) in the plasma membrane confirmed by a cell surface biotinylation assay. It was performed 48 h after HEK293 cells were transiently transfected with an expression vector containing mutated (I406AQ407A) or wild type (wt) or no (empty vector) hsTRPM2. The membrane for Western Blotting was cut in between the 140 kDa and 95 kDa marker band to separately treat the membrane parts with either anti-hsTRPM2 or anti-Na+/K+-ATPase primary antibody. The shown image represents a grouped image of the two membrane parts. Only detected bands are shown, not full scans of the membrane parts. (C) Binding of Ca2+-Calmodulin to peptides containing the wild type (wt RK26) or mutated (I406AQ407A, mut RK26) N-terminal IQ-like motif confirmed by isothermal titration calorimetry (ITC). KD values are given as means ± SEM of n = 3 experiments.