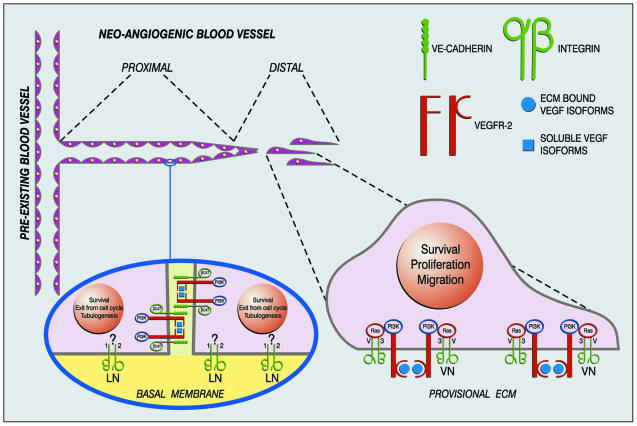
Fig. 1. A working hypothesis for endothelial transmembrane signaling modules during sprouting angiogenesis. When a neo-angiogenic sprout buds from a pre-existing blood vessel, ECs localized at the tip of the sprout proliferate and migrate, whereas those more proximal to the pre-existing blood vessel exit from the cell cycle and take the shape of stable and functional capillaries. In the basal membrane domain integrins mediate adhesion of EC to the ECM. In the lateral membrane domain cadherins mediate cell–cell interactions. At the distal tip cell–cell contacts are absent or transient, whereas in the proximal section of the neo-vessel, firm and stable intercellular contacts are mandatory for a proper tubuligenesis. At the distal tip, within the VEGFR-2-αvβ3 signaling module, adhesive interaction of vitronectin with αvβ3 allows VEGFR-2 interactions with ECM-bound VEGF isoforms switching on a proliferative, migratory and survival program. In the proximal vessel portion, in the context of the VEGFR-2-VE-cadherin signaling module, homophilic interactions between VE-cadherin permit VEGFR-2 binding to soluble VEGF isoforms, which induces maturation, remodeling and long-term survival of stabilized EC tubes. In addition, whereas adhesion to vitronectin through the Shc-linked integrin αvβ3 promotes proliferation, adhesion to laminin of basal lamina through the non-Shc-linked α2β1 integrin promotes exit from the cell cycle and co-operates with VE-cadherin to the formation of endothelial tubular structures (Languino et al., 1989).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.