Abstract
Background:
Prenatal exposure to metals is hypothesized to be associated with child autism. We aim to investigate the joint and individual effects of prenatal exposure to urine metals including lead (Pb), mercury (Hg), manganese (Mn), and selenium (Se) on child Social Responsiveness Scale (SRS) scores.
Methods:
We used data from 2 cohorts enriched for likelihood of autism spectrum disorder (ASD): Early Autism Risk Longitudinal Investigation (EARLI) and the Markers of Autism Risk in Babies-Learning Early Signs (MARBLES) studies. Metal concentrations were measured in urine collected during pregnancy. We used Bayesian Kernel Machine Regression and linear regression models to investigate both joint and independent associations of metals with SRS Z-scores in each cohort. We adjusted for maternal age at delivery, interpregnancy interval, maternal education, child race/ethnicity, child sex, and/or study site.
Results:
The final analytic sample consisted of 251 mother-child pairs. When Pb, Hg, Se, and Mn were at their 75th percentiles, there was a 0.03 increase (95% credible interval [CI]: −0.11, 0.17) in EARLI and 0.07 decrease (95% CI: −0.29, 0.15) in MARBLES in childhood SRS Z-scores, compared to when all 4 metals were at their 50th percentiles. In both cohorts, increasing concentrations of Pb were associated with increasing values of SRS Z-scores, fixing the other metals to their 50th percentiles. However, all the 95% credible intervals contained the null.
Conclusions:
There were no clear monotonic associations between the overall prenatal metal mixture in pregnancy and childhood SRS Z-scores at 36 months. There were also no clear associations between individual metals within this mixture and childhood SRS Z-scores at 36 months. The overall effects of the metal mixture and the individual effects of each metal within this mixture on offspring SRS Z-scores might be heterogeneous across child sex and cohort. Further studies with larger sample sizes are warranted.
Keywords: Mixture, ASD, BKMR
Introduction
Autism spectrum disorder (ASD) is an increasingly recognized public health issue. The prevalence of ASD diagnoses in the United States among children aged 8 years has increased from one in 150 children in 2000 to one in 36 children in 2020.1,2 There are substantial health burdens and financial costs associated with ASD. Over 95% of children with ASD had at least one comorbidity, such as epilepsy or language disorder. 3 Adults with ASD had higher rates of psychiatric disorders, such as depression and schizophrenia, compared to adults without ASD. 4 In the United States, the lifetime cost of supporting one individual with ASD was $1.4 to $2.4 million in 2011. 5
ASD is characterized by 2 sets of core features: impaired social communication and social interaction, and restricted and repetitive behaviors and interests.6,7 Developmental traits related to ASD, also known as quantitative autistic traits or simply autistic traits, are defined as the subclinical manifestation of these characteristics that can be measured on a continuous scale, forming a spectrum among both the general population and individuals with ASD.8-10 There are multiple tools for screening autistic traits, such as the social responsiveness scale (SRS), which is a widely utilized and well-validated instrument for measuring the presence and severity of impaired social communication and social interaction. 11 While ASD is considered one of the most heritable neurodevelopmental disorders, with estimated heritability of 64% to 91%, 12 environmental factors have been attracting more research interests because of an ever-expanding body of evidence for neurotoxicity, including associations with ASD, of many such exposures, for example, pesticides, phthalates, air pollution, and because they are amenable to interventions. 13 In particular, metals have been found to be associated with increased likelihood of autism or autistic traits (eg, decreased social interaction with peers and increased repetitive behaviors) in both animal experiments14,15 and epidemiological studies.16-20 For example, Roberts et al showed that perinatal exposures to individual metals from ambient air pollution, including lead (Pb), mercury (Hg), and manganese (Mn), was associated with higher odds of ASD among children born after 1987 in the United States. 17 Yoshimasu et al also found that prenatal/early infancy exposures to ambient Hg was associated with higher odds of ASD by conducting a meta-analysis including 3 individual studies of children born from 1987 to 2002. 21 Another study demonstrated that higher body burden of toxic metals, including Pb and Hg in urine, was associated with greater severity of ASD among children aged 3 to 8 years old. 22 On the other hand, one ecological study found a lack of association between air Hg and ASD prevalence in Texas. 23 Results from the Early Markers for Autism (EMA) Study showed that neither maternal nor neonate blood Hg was associated with ASD among children born in 2000–2001. 24 Nevertheless, Kern et al concluded in their review article that Hg from various biological matrices (eg, blood and urine) contributed to the etiology of ASD. 25 A detailed summary of the epidemiologic evidence has been published elsewhere. 26
There are a multitude of metals that exist in both natural and man-made environments, among which trace metals are a group of metals that are present in living organisms at very low concentrations. 27 These metals can be classified into 2 categories: essential metals, which have certain physiological functions for human bodies at certain levels, and non-essential metals, which are not needed for human bodies. 28 Examples of essential metals include Mn, selenium (Se), zinc, and iron, whereas non-essential metals include Pb, Hg, and cadmium (Cd).29,30 Despite the progress made in environmental policies in the past decades, it was estimated that nearly 50% of participants from the 2007 to 2012 National Health and Nutrition Examination Survey (NHANES) had at least 3 of Pb, Hg, Cd, and/or arsenic (As) present in their bodies. 31 Additionally, an analysis of data from the NHANES 2003-2004 cycle estimated that 66% to 99% of women of reproductive age in the United States had detectable levels of Pb, Hg, and Cd in their blood. 32
During pregnancy, these metals can cross the placenta and the blood–brain barrier (BBB), affecting the babies’ developing brains via multiple mechanisms. 33 For example, Pb disrupts the BBB by mimicking or mobilizing calcium and protein kinase C, 34 Hg diffuses across the BBB, 35 Mn can readily cross BBB 36 or cross the BBB mediated by a transferrin receptor, 36 Cd increases BBB permeability, 37 and Se can be transported into the brain by various receptors such as megalin. 38 These heavy metals are well-known neurotoxicants that can cause a wide range of disorders and diseases. 39 Pb is notorious for its negative impact on intelligence quotient (IQ) and other learning abilities.39-42 Studies have also shown that Pb can induce Alzheimer’s disease-like pathogenesis by altering synaptic plasticity and disrupting calcium homeostasis.43,44 The consequences of Hg poisoning were underscored by the Minamata disaster in Japan. 45 Cd has also been linked to decreased IQ and other learning abilities. 37 On the other hand, Se and Mn can either protect or harm fetal neurodevelopment, depending on the doses and co-occurrence of other metals.46-49 Specifically, both high and low levels of prenatal Se concentrations had adverse impact on child neuropsychological and neurobehavioral development, forming an inverted U-shaped association.50,51 Similarly, an inverted U-shaped association was also observed between prenatal blood Mn levels and child neurobehavioral outcomes, 52 with implications of Mn in oxidative stress53,54 and neurotransmitter metabolism.55-57
Notably, the potential associations between metals exposures and ASD-related outcomes may be modified by child sex at birth. 58 For example, the associations between perinatal exposures to multiple metals from ambient air pollution and ASD were significantly stronger among boys compared to girls. 17 Additionally, boys are over 4 times more likely to be diagnosed with ASD compared to girls in the United States. 2 Finally, it has been observed that girls exhibit less severe autistic traits in comparison to boys.59,60 Therefore, there has been a considerable need to investigate the potential effect measure modification of child sex in the associations between metals exposures and ASD-related outcomes using epidemiological data as the current evidence is scarce. 58
To date, most epidemiological studies have focused on a single metal exposure, and few have investigated metal mixtures and their potential impact on quantitative traits related to ASD. While a single metal at a low concentration may have minimal health effects, they may impose greater negative health impacts on human bodies as a mixture. 61 Furthermore, multiple metals within a mixture may have non-linear and non-additive associations with a health outcome. 62 Some key limitations have been the lack of prospective study, limited sample sizes, failure to include quantitative measures of ASD other than a dichotomous diagnosis, and imprecise metal measurements during ASD-related windows of susceptibility.
This study aims to address these limitations by using both Bayesian and frequentist methods to explore the relationship between prenatal exposure to multiple metals and autistic traits using data from the Early Autism Risk Longitudinal Investigation (EARLI) and the Markers of Autism Risk in Babies-Learning Early Signs (MARBLES) cohort. Our research questions include whether: a) there is an association between a mixture of urine metals (Pb, Hg, Se, and Mn) and autistic traits; and b) there are associations between individual metals within the metal mixture and autistic traits, accounting for the other co-occurring metals.
Methods
Participants
Data were compiled from 2 longitudinal cohorts enriched for probability of ASD—the Early Autism Risk Longitudinal Investigation (EARLI) and the Markers of Autism Risk in Babies—Learning Early Signs (MARBLES) cohort. Details of these 2 cohorts have been published elsewhere.63,64 Briefly, the EARLI cohort enrolled 246 pregnant women > 18 years old who previously had a child with ASD at sites at Drexel University, Johns Hopkins University, Kaiser Permanente Northern California, and University of California Davis. 63 Biological samples were collected during first and second trimesters. 63 The MARBLES cohort enrolled 361 pregnant women who were > 18 years old, carrying offspring who would have a half-sibling, or an equivalent or closer blood relative, with an ASD diagnosis, and who lived within 2.5 hours’ drive from the University of California Davis MIND Institute in the Davis/Sacramento area. 64 All the children, that is, the offspring of those pregnancies, were born between November 2009 and March 2012 in EARLI and between December 2006 and July 2016 in MARBLES. 65 These siblings of autistic children had 20-fold higher risk of having ASD themselves compared to the general population and were thus at “enriched risk” of ASD, providing a unique opportunity to study the complex etiology of ASD with an increased sample size from both cohorts. 66 Biological samples were collected throughout pregnancy. 64 Both cohorts also obtained behavioral assessments of children up to 36 months to enable ASD diagnosis and scoring of ASD-related quantitative traits (73 and 226 children completed the SRS assessment, respectively).
Outcome measures
We quantified deficits in social behavior associated with ASD using the SRS, a questionnaire with 65 items.67,68 Each of the items were scored on a 4-point Likert scale (0 = not true, 1 = sometimes true, 2 = often true, and 3 = almost always true).67,68 The SRS questionnaires were administered to parents at 36-month visits in EARLI, or at older ages for participants enrolled in MARBLES in earlier years. 69 We calculated the total scores by summing the scores of the 65 individual items (scoring range: 0-195), with higher raw scores indicating greater social communication deficits related to ASD. The interrater reliability and retest reliability of the SRS were between 0.76 and 0.95 and between 0.84 and 0.97, respectively. 70 And the convergent validity of the SRS against the Autism Diagnostic Interview—Revised (ADI-R), the Autism Diagnostic Observation Schedule (ADOS), and the Social Communication Questionnaire (SCQ) was between 0.35–0.58. 70 We then calculated SRS Z-scores by subtracting the total raw score by cohort-specific mean then divided by cohort-specific standard deviation. For this analysis, we included the mother-singleton child pairs with non-missing prenatal urinary metals and Social Responsiveness Scale (SRS) scores at 36 months.
Exposure measures
The National Sanitation Foundation (NSF) International (Ann Arbor, Michigan, United States) analyzed maternal urine samples to quantify prenatal exposure to metals using inductively coupled plasma mass spectrometry (ICP-MS) based on the Centers for Disease Control and Prevention (CDC) Method No. 3018.3, 71 with modifications for the expanded metals panel and the Thermo Scientific™ iCAP™ RQ instrument (Serial number RQ0029). All urine samples, standards, quality controls, and blanks were diluted 10-fold in a diluent consisting of 2% HNO3 solution containing the internal standards and gold standards. The diluent was also the rinse solution for the instrument. The urine samples were then analyzed in kinetic energy discrimination (KED) mode for cadmium and in standard (default) mode for the other metals.
All metals were measured in parts per billion (ppb), that is, micrograms per liter (µg/L). We imputed values below the lower limit of detections (LLODs) with LLOD divided by the square root of two.72,73 Values above the LLODs remained unchanged. Since less than 60% of the cadmium concentrations were above the LLODs in both cohorts, we excluded it from further analyses. We then corrected all the metal measurements for urinary dilution using specific gravity such that , where was the urinary metal concentration adjusted for urinary specific gravity, was the raw urinary metal concentration, was the median specific gravity across all samples, and was the observed urinary specific gravity value for each urine sample.74,75 This approach has been widely used in epidemiologic studies and produced similar performance as other dilution adjustment methods for urinary biomarkers. 76 To facilitate interpretation, we log2-transformed all the metals after correcting for the values below the LLODs and urinary dilution. Due to the limited sample sizes in each pregnancy trimester, we calculated the average metals levels across pregnancy for each mom (Supplemental Table 1). For example, there were 120 pregnant women with metals data for the first and third trimesters in EARLI and the average of these 2 values at the 2 time points was calculated for each pregnant woman (Supplemental Table 1). Overall, 143 (93%) and 69 (71%) pregnant women had 2 measures averaged in EARLI and MARBLES, respectively (Supplemental Table 1).
Covariates
We identified covariates a priori that had been identified as important factors related to ASD from the existing literature (Supplemental Figure 1). Based on data availability, we selected the following covariates of interest: maternal age at delivery (years), interpregnancy interval (months), maternal education (less than high school, high school diploma/General Educational Development [GED], some college, Bachelor’s degree, Graduate or professional degree), child race/ethnicity (white, Black/African American, Asian, other race, multiracial), child sex (male, female), and study site (Drexel University, Johns Hopkins University, Kaiser Permanente Northern California, University of California Davis, Davis/Sacramento area).
Statistical analyses
We described continuous variables using medians (minimums, maximums) and categorical variables using counts (percentages). We fitted Bayesian Kernel Machine Regression (BKMR) models to estimate a single summed effect of the metal mixture (Pb, Hg, Se, and Mn) as well as individual effects of each metal on SRS Z-scores using the R packages “bkmr” (version 0.2.0)62,77,78 and “bkmrhat” (version 1.1.1).79,80 The BKMR model was defined as: , where was a monotone link function, µi was the expected value of the SRS scores for individuals , was a flexible function that allowed for non-linearity of dose-response curves and interaction among the mixture components, were the chemical concentrations for individual , were a vector of the covariates for individual and were the corresponding vector of coefficients.62,81 We used the kmbayes_parallel function from the “bkmrhat” package to fit a BKMR model using 4 Markov chain Monte Carlo (MCMC) parallel chains with 10 000 iterations for each chain, including 1000 burn-in iterations per chain. 79 We performed model diagnostics on the 4 MCMC chains by converting the BKMR fits to mcmc.list objects from the “coda” package using the as.mcmc.list function from the “bkmrhat” package. 79 We defined model convergence as Gelman’s r-hat diagnostic = 1.0 and effective sample size (ESS) > 100. 82 We summarized model output after combining the chains from the original kmbayes_parallel fit into a single chain by using the kmbayes_combine function.
We also constructed multiple linear regression models according to the following steps: 1) we fitted a saturated model with the SRS Z-scores as the dependent variable, and log2-transformed metal concentrations, covariates defined as above, and 2-way interaction terms of each pair of the metals as the independent variables; 2) we conducted a likelihood ratio test and a F-test to decide whether to keep the 2-way interaction terms of each pair of the metals as the independent variables with the imprecise hypothesis of all the beta coefficients for the interactions terms equal to zero; and 3) we assessed the appropriateness of the mean function by visually inspecting the residuals against each of the metals and the residuals against predicted SRS Z-scores plots. The independence assumption was assumed not violated because there was one average concentration of each of the metals for each pregnant woman, and the main sources for the different metals examined in this analysis vary considerably. We checked the constant variance assumptions by visually inspecting the squared residuals against each of the metals. The SRS was modeled using Z-scores to be consistent with previous study utilizing the EARLI data and similar Bayesian methods. 83 We conducted sex-stratified analyses to assess the potential effect measure modification by child sex at birth.
We performed all the data management, data analyses except the BKMR models, and data visualization using R version 4.2.0 (2022-04-22) under a macOS Big Sur/Monterey 10.16 system. We fitted the BKMR models using R version 4.2.3 (2023-03-15) under a Windows 10 x64 system.
Ethics statement
The EARLI study was reviewed and approved by Human Subjects Institutional Review Boards (IRBs) from each of the 4 EARLI study sites (Johns Hopkins University, Drexel University, University of California Davis, and Kaiser Permanente) and the MARBLES study was reviewed and approved by the Human Subjects IRB from University of California Davis. 65 This secondary analysis was reviewed and approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board (IRB number: IRB00002032).
Code and data availability
Data requests should be addressed to the Principal Investigators of the 2 cohorts (EARLI: MDF; MARBLES: RJS). After the paper is accepted for publication, the code will be made publicly available at https://github.com/emmayu001/BKMR-Metals-SRS.
Results
Descriptive statistics
There were 246 and 361 pregnant women enrolled in the EARLI and MARBLES cohorts, respectively (Supplemental Figure 2). After excluding twin births and mother-child pairs with missing metals measures or SRS Z-scores, we reached a final analytic sample size of 251 mother-child pairs including 154 mother-child pairs from EARLI and 97 mother-child pairs from MARBLES (Supplemental Figure 2). The sociodemographic characteristics of the mother-child dyads were comparable between EARLI and MARBLES (Table 1). Overall, the median age at delivery was 34 years old, the median interpregnancy interval was 45.3 months, and over 85% of the mothers had at least some college education (Table 1). Among the singleton births, EARLI had 46.8% (n = 72) females while MARBLES had 50.5% (n = 49) females (Table 1). The medians (minimums, maximums) of SRS total raw scores among the children at 36 months were 28.0 (6.0, 174.0) and 34.0 (2.0, 139.0) for EARLI and MARBLES, respectively (Table 1).
Table 1.
Sociodemographic characteristics of the analytic sample (N = 251).
| EARLI (N = 154) | MARBLES (N = 97) | Pooled (N = 251) | |
|---|---|---|---|
| Maternal characteristics | |||
| Maternal age at delivery, years | 34.0 (22.0, 44.0) | 34.3 (20.5, 47.1) | 34.0 (20.5, 47.1) |
| Interpregnancy interval, months a | 44.6 (1.4, 161.0) | 46.9 (8.8, 150.0) | 45.3 (1.4, 161.0) |
| Mother’s education | |||
| High school diploma/GED or below | 20 (13.0%) | 10 (10.3%) | 30 (11.9%) |
| Some college | 40 (26.0%) | 37 (38.1%) | 77 (30.7%) |
| Bachelor’s degree | 47 (30.5%) | 25 (25.8%) | 72 (28.7%) |
| Graduate or professional degree | 44 (28.6%) | 25 (25.8%) | 69 (27.5%) |
| Missing | 3 (1.9%) | 0 (0%) | 3 (1.2%) |
| Child characteristics | |||
| Child sex | |||
| Female | 72 (46.8%) | 49 (50.5%) | 121 (48.2%) |
| Male | 82 (53.2%) | 48 (49.5%) | 130 (51.8%) |
| Child race/ethnicity | |||
| White (European/Middle Eastern descent) | 82 (53.2%) | 54 (55.7%) | 136 (54.2%) |
| Black/African American | 12 (7.8%) | 3 (3.1%) | 15 (6.0%) |
| Asian | 10 (6.5%) | 13 (13.4%) | 23 (9.2%) |
| Other race (not specified) | 10 (6.5%) | 0 (0%) | 10 (4.0%) |
| Multiracial/Hispanic | 32 (20.8%) | 27 (27.8%) | 59 (23.5%) |
| Missing | 8 (5.2%) | 0 (0%) | 8 (3.2%) |
| SRS total raw score | 28.0 (6.0, 174.0) | 34.0 (2.0, 139.0) | 30.0 (2.00, 174) |
| SRS total T-score | 49.0 (38.0, 97.0) | 47.0 (35.0, 87.0) | 48.0 (35.0, 97.0) |
| SRS Z-score | −0.289 (−1.09, 5.00) | −0.291 (−1.34, 3.14) | −0.289 (−1.34, 5.00) |
| Study site b | |||
| Drexel | 39 (25.3%) | 0 (0%) | 39 (15.5%) |
| Johns Hopkins University | 38 (24.7%) | 0 (0%) | 38 (15.1%) |
| Kaiser Permanente | 51 (33.1%) | 0 (0%) | 51 (20.3%) |
| UC Davis (EARLI site) | 26 (16.9%) | 0 (0%) | 26 (10.4%) |
| Davis/Sacramento area (MARBLES site) | 0 (0%) | 97 (100%) | 97 (38.6%) |
Abbreviations: EARLI, Early Autism Risk Longitudinal Investigation; GED, General Educational Development; HS, high school; MARBLES, Markers of Autism Risk in Babies Learning the Early Signs; SD, standard deviation; SRS, Social Responsiveness Scale.
All values are medians (minimums, maximums) unless otherwise specified.
Interpregnancy interval (IPI) was defined as the number of months between last parity pregnancy to last menstrual period for child of interest. IPI had 6 missing values (4 from EARLI and 2 from MARBLES) and we used multiple imputation by chained equations to impute these missing values and missing values in the other covariates.
While EARLI and MARBLES both had a recruitment site in the Davis/Sacramento area, the same pregnant woman could only be enrolled in one of the, but not both, cohorts.
The distributions of log2-transformed metals were comparable in EARLI and MARBLES (Table 2; Figure 1). Spearman correlation coefficients of each metal between different trimesters and between each pair of metals ranged from −.2 to .5 in EARLI and ranged from −.2 to .7 in MARBLES, suggesting moderate correlations between the metals and warranting a mixture approach (Supplemental Figure 3). Spearman correlation coefficients of SRS Z-scores and each pair of metals across the pregnancy ranged from −.01 to .38 in EARLI and ranged from −.01 to .54 in MARBLES (Supplemental Figure 4).
Table 2.
Average concentrations of urinary metals throughout pregnancy in the analytic sample (N = 251).
| Metal, µg/L | Mean (SD) | Median (Min, Max) | LLOD a | N (%) ≥ LLOD b |
|---|---|---|---|---|
| Lead | ||||
| EARLI (N = 154) | 0.28 (0.24) | 0.24 (0.04, 2.50) | 0.10, 0.20 | 101 (65.6%) |
| MARBLES (N = 97) | 0.28 (0.24) | 0.22 (0.05, 1.70) | 0.10, 0.20 | 40 (41.2%) |
| Mercury | ||||
| EARLI (N = 154) | 0.25 (0.21) | 0.19 (0.03, 1.70) | 0.05, 0.10 | 139 (90.3%) |
| MARBLES (N = 97) | 0.29 (0.77) | 0.15 (0.04, 7.60) | 0.05, 0.10 | 62 (63.9%) |
| Cadmium | ||||
| EARLI (N = 154) | 0.15 (0.12) | 0.12 (0.03, 0.95) | 0.06, 0.12 | 82 (53.2%) |
| MARBLES (N = 97) | 0.13 (0.09) | 0.10 (0.03, 0.53) | 0.06, 0.12 | 28 (28.9%) |
| Selenium | ||||
| EARLI (N = 154) | 39.4 (15.8) | 35.9 (13.3, 135) | 2.00, 4.00 | 154 (100%) |
| MARBLES (N = 97) | 39.1 (15.0) | 36.7 (16.0, 114) | 2.00, 4.00 | 97 (100%) |
| Manganese | ||||
| EARLI (N = 154) | 0.29 (0.17) | 0.26 (0.05, 1.00) | 0.08, 0.16 | 130 (84.4%) |
| MARBLES (N = 97) | 0.31 (0.16) | 0.27 (0.07, 1.00) | 0.08, 0.16 | 72 (74.2%) |
Abbreviations: EARLI, Early Autism Risk Longitudinal Investigation; LLOD, lower limit of detection; MARBLES, Markers of Autism Risk in Babies Learning the Early Sign.
We imputed metal concentrations below the lower limit of detection (LLOD) with the LLOD divided by square root of 2 and then corrected for urinary specific gravity.
We list 2 LLODs for each metal here since there were differences in LLODs by batch.
Number (percentage) of moms with at least one sample ≥ LLOD.
Figure 1.
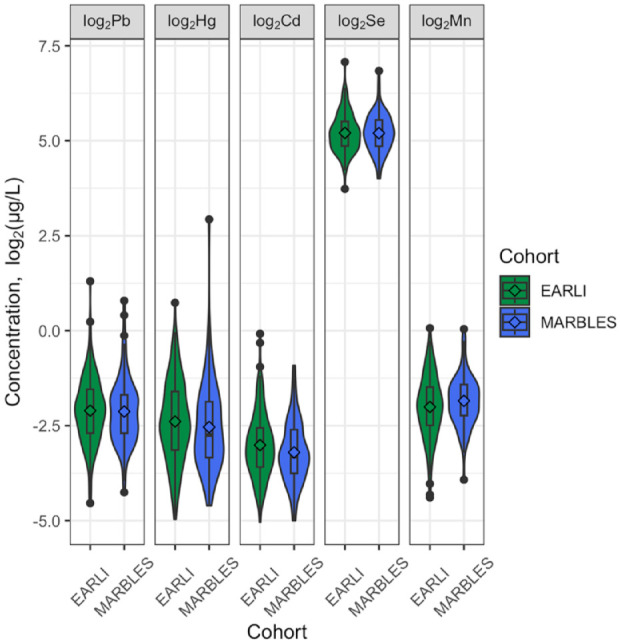
Profiles of maternal urinary metals during pregnancy, corrected for urinary specific gravity (N = 251). Cd, cadmium; EARLI, Early Autism Risk Longitudinal Investigation; Hg, mercury; MARBLES, Markers of Autism Risk in Babies Learning the Early Signs; Mn, manganese; Pb, lead; Se, selenium.
Model selection and diagnostics
The MCMC diagnostics indicated good convergence for all the parameters with Gelman’s r-hat diagnostics equal to 1.0 and effective sample sizes > 100. For the linear regression models, both the likelihood ratio test and the F-test indicated that there was not enough evidence to reject the null hypothesis of no interaction between the metals. Hence, we removed the 2-way interaction terms of each pair of the metals. In consideration of the sample size and to facilitate interpretation, parsimony, and comparison with the BKMR results, we did not pursue further investigation of those interactions.
Overall effects of the metal mixture on SRS Z-scores (BKMR)
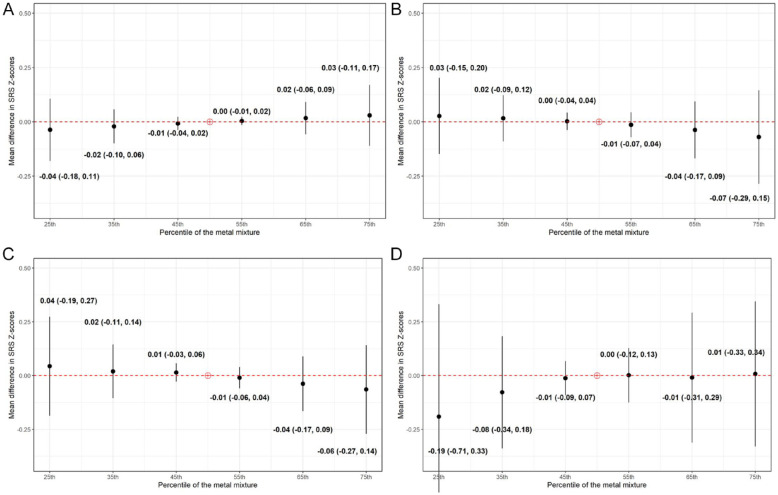
Overall, SRS Z-scores increased with higher mixture percentiles in EARLI, whereas they decreased with higher mixture percentiles in MARBLES (Figure 2), although both associations were weak. There was a 0.03 increase (95% credible interval [CI]: −0.11, 0.17) in EARLI and 0.07 decrease (95% CI: −0.29, 0.15) in MARBLES in mean SRS Z-scores when all Pb, Hg, Se, and Mn were at their 75th percentiles (Figure 2), compared to when Pb, Hg, Se, and Mn were at their 50th percentiles. However, both 95% credible intervals contained the null (Figure 2). When we stratified the pooled data from the 2 cohorts by child sex at birth, we observed a downward trend among male children but a less clear pattern in female children (Figure 2).
Figure 2.
Overall effects of the metal mixture (ie, lead, mercury, selenium, and manganese) during pregnancy on offspring SRS-2 Z-scores by cohort and child sex in Bayesian Kernel Machine Regression models comparing the values of SRS-2 Z-scores when all the metals are at a particular percentile as compared to when all of them are at their 50th percentiles: (A) EARLI, (B) MARBLES, (C) males, and (D) females.
Contribution of individual metals within the mixture to SRS Z-scores
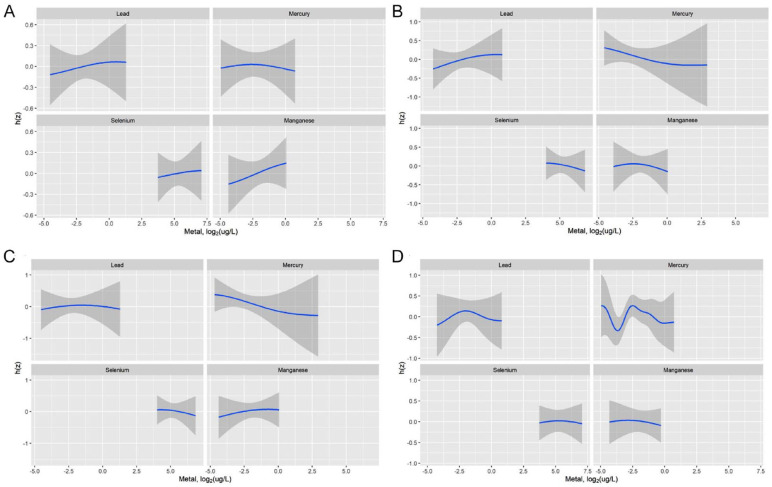
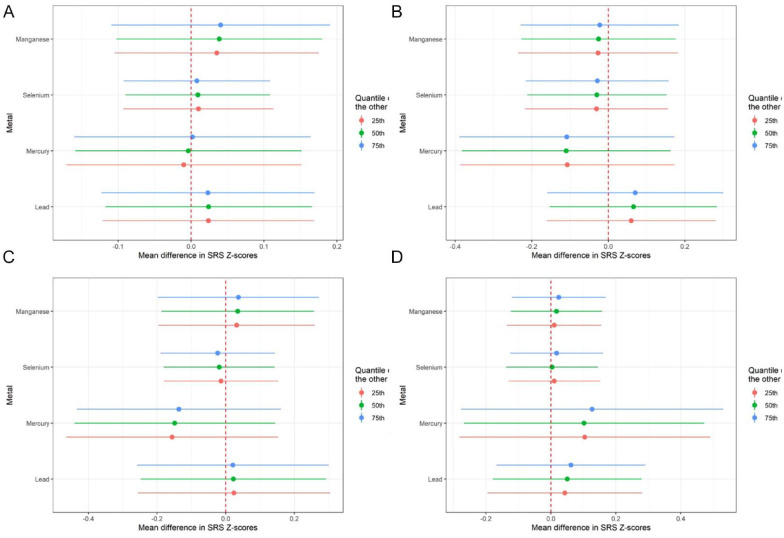
In both EARLI and MARBLES, increasing concentrations of Pb were associated with increasing values of SRS Z-scores, fixing the other metals to their 50th percentiles and holding all the covariates constant (Figures 3 and 4). Results for the other metals were less consistent between the 2 cohorts (Figure 3). For example, increasing concentrations of Se was associated with increasing values of SRS Z-scores in EARLI but with decreasing values of SRS Z-scores in MARBLES (Figures 3 and 4). When we stratified the data by child sex, Pb and Se showed inverted-U shape relationships with SRS Z-scores (Figures 3 and 4). Again, all the 95% credible intervals for the pointe estimates contained the null (Figures 3 and 4).
Figure 3.
Metal-Social Responsiveness Scale (SRS-2) Z-score exposure-response function by cohort and child sex from Bayesian Kernel Machine Regression model. h(z) is the exposure-response function: (A) EARLI, (B) MARBLES, (C) males, and (D) females.
Figure 4.
Single-exposure effects of each metal within the mixture (ie, lead, mercury, selenium, and manganese) during pregnancy on offspring Social Responsiveness Scale (SRS-2) Z-scores by cohort and child sex in Bayesian Kernel Machine Regression model: (A) EARLI, (B) MARBLES, (C) males, and (D) females.
Estimates from the multiple linear regression model were consistent with the results from the BKMR models as there were no clear associations between the SRS Z-scores and a doubling of the prenatal Pb, Hg, Se, or Mn (Tables 3 and 4). When modeling the metals using their quartiles, the results were overwhelmingly null from the multiple linear regression models in both cohorts and both child sex groups as well (Tables 3 and 4).
Table 3.
Associations between individual prenatal metals concentrations and offspring Social Responsiveness Scale (SRS-2) Z-scores at 36 months by cohort: linear regression models.
| Metal and levels, log2(µg/L) | EARLI a (N = 154) | MARBLES b (N = 97) | ||
|---|---|---|---|---|
| Range | Beta (95% CI) | Range | Beta (95% CI) | |
| Lead | ||||
| First quartile | −4.5 to −2.7 | Reference | −4.3 to −2.7 | Reference |
| Second quartile | −2.7 to −2.1 | 0.49 (0.02, 0.96) | −2.7 to −2.2 | −0.33 (−0.96, 0.30) |
| Third quartile | −2.1 to −1.5 | 0.24 (−0.23, 0.71) | −2.2 to −1.7 | 0.18 (−0.50, 0.85) |
| Fourth quartile | −1.5-1.3 | 0.27 (−0.24, 0.77) | −1.7-0.8 | 0.12 (−0.60, 0.85) |
| A doubling in concentration | — | 0.01 (−0.20, 0.21) | — | 0.16 (−0.12, 0.43) |
| Mercury | ||||
| First quartile | −5.0 to −3.1 | Reference | −4.6 to −3.3 | Reference |
| Second quartile | −3.1 to −2.4 | −0.15 (−0.61, 0.31) | −3.3 to −2.7 | 0.13 (−0.50, 0.76) |
| Third quartile | −2.4 to −1.6 | −0.34 (−0.81, 0.13) | −2.7 to −1.9 | −0.38 (−1.03, 0.26) |
| Fourth quartile | −1.6-0.7 | −0.19 (−0.66, 0.29) | −1.9-2.9 | −0.07 (−0.71, 0.57) |
| A doubling in concentration | — | −0.03 (−0.19, 0.12) | — | −0.08 (−0.28, 0.12) |
| Selenium | ||||
| First quartile | 3.7-4.9 | Reference | 4.0-4.9 | Reference |
| Second quartile | 4.9-5.2 | 0.20 (−0.25, 0.65) | 4.9-5.2 | −0.05 (−0.67, 0.57) |
| Third quartile | 5.2-5.5 | 0.27 (−0.20, 0.74) | 5.2-5.5 | −0.01 (−0.61, 0.59) |
| Fourth quartile | 5.5-7.1 | 0.18 (−0.28, 0.64) | 5.5-6.8 | 0.00 (−0.63, 0.62) |
| A doubling in concentration | — | 0.10 (−0.22, 0.42) | — | −0.13 (−0.54, 0.28) |
| Manganese | ||||
| First quartile | −4.4 to −2.5 | Reference | −3.9 to −2.2 | Reference |
| Second quartile | −2.5 to −2.0 | 0.11 (−0.37, 0.60) | −2.2 to −1.9 | 0.27 (−0.35, 0.89) |
| Third quartile | −2.0 to −1.5 | −0.03 (−0.50, 0.44) | −1.9 to −1.4 | 0.31 (−0.31, 0.94) |
| Fourth quartile | −1.5-0.1 | 0.34 (−0.16, 0.84) | −1.4-0.04 | −0.07 (−0.73, 0.59) |
| A doubling in concentration | — | 0.15 (−0.06, 0.36) | — | 0.01 (−0.33, 0.35) |
All the values are regression coefficients (95% confidence intervals) unless otherwise specified. We imputed metal concentrations below the lower limit of detection (LLOD) with the LLOD divided by square root of 2 and then corrected for urinary specific gravity.
The 2 models included the 4 urine metals (log2-transformed; continuous or quartiles), maternal age at delivery (years), interpregnancy interval (months), 4 dummy variables for maternal education, 3 dummy variables for child race, child sex, and 3 dummy variables for study site.
The 2 models included the 4 urine metals (log2-transformed; continuous or quartiles), maternal age at delivery (years), interpregnancy interval (months), 4 dummy variables for maternal education, 3 dummy variables for child race, and child sex.
Table 4.
Associations between individual prenatal metals and offspring Social Responsiveness Scale (SRS-2) Z-scores at 36 months by child sex: linear regression models.
| Metal and levels, log2(µg/L) | Males a (N = 130) | Females a (N = 121) | ||
|---|---|---|---|---|
| Range | Beta (95% CI) | Range | Beta (95% CI) | |
| Lead | ||||
| First quartile | −4.5 to −2.8 | Reference | −4.3 to −2.6 | Reference |
| Second quartile | −2.8 to −2.2 | 0.47 (−0.16, 1.11) | −2.6 to −2.0 | −0.02 (−0.45, 0.41) |
| Third quartile | −2.2 to −1.6 | −0.12 (−0.75, 0.51) | −2.0 to −1.7 | 0.07 (−0.34, 0.49) |
| Fourth quartile | −1.6-1.3 | 0.30 (−0.38, 0.99) | −1.7-0.8 | 0.05 (−0.40, 0.51) |
| A doubling in concentration | — | 0.05 (−0.23, 0.33) | — | 0.11 (−0.08, 0.29) |
| Mercury | ||||
| First quartile | −4.7 to −3.2 | Reference | −5.0 to −3.3 | Reference |
| Second quartile | −3.2 to −2.4 | −0.25 (−0.87, 0.37) | −3.3 to −2.6 | 0.37 (−0.04, 0.78) |
| Third quartile | −2.4 to −1.7 | −0.89 (−1.52, −0.27) | −2.6 to −1.8 | 0.52 (0.10, 0.94) |
| Fourth quartile | −1.7-2.9 | −0.43 (−1.07, 0.21) | −1.8-0.7 | 0.26 (−0.15, 0.67) |
| A doubling in concentration | — | −0.15 (−0.36, 0.06) | — | 0.00 (−0.14, 0.14) |
| Selenium | ||||
| First quartile | 4.0-4.8 | Reference | 3.7-4.9 | Reference |
| Second quartile | 4.8-5.1 | 0.38 (−0.19, 0.96) | 4.9-5.2 | 0.05 (−0.37, 0.48) |
| Third quartile | 5.1-5.4 | −0.04 (−0.62, 0.55) | 5.2-5.6 | 0.18 (−0.23, 0.58) |
| Fourth quartile | 5.4-6.8 | 0.41 (−0.17, 1.00) | 5.6-7.1 | 0.19 (−0.23, 0.60) |
| A doubling in concentration | — | 0.04 (−0.40, 0.47) | — | 0.02 (−0.25, 0.29) |
| Manganese | ||||
| First quartile | −4.4 to −2.4 | Reference | −4.3 to −2.4 | Reference |
| Second quartile | −2.4 to −1.9 | 0.28 (−0.32, 0.87) | −2.4 to −2.0 | 0.03 (−0.40, 0.46) |
| Third quartile | −1.9 to −1.4 | 0.36 (−0.27, 0.99) | −2.0 to −1.5 | 0.16 (−0.25, 0.58) |
| Fourth quartile | −1.4-0.1 | 0.02 (−0.62, 0.67) | −1.5 to −0.3 | −0.01 (−0.46, 0.44) |
| A doubling in concentration | — | 0.07 (−0.21, 0.35) | — | 0.08 (−0.15, 0.30) |
All the values are regression coefficients (95% confidence intervals) unless otherwise specified. We imputed metal concentrations below the lower limit of detection (LLOD) with the LLOD divided by square root of 2 and then corrected for urinary specific gravity.
The 2 models included the 4 urine metals (log2-transformed; continuous or quartiles), maternal age at delivery (years), interpregnancy interval (months), 4 dummy variables for maternal education, 3 dummy variables for child race, and 4 dummy variables for study site.
Discussion
To our knowledge, this study is one of the first studies to directly evaluate the associations between prenatal exposure to a metal mixture and later offspring SRS Z-scores, a quantitative phenotype of ASD.8,26 Leveraging a pooled sample of the EARLI and MARBLES pregnancy cohorts with enriched familial likelihood for ASD, we found that: a) exposure to a metal mixture (Pb, Hg, Se, and Mn) during windows of susceptibilities was not monotonically associated with higher offspring SRS Z-scores at 36 months in EARLI or MARBLES; b) there were no clear associations between individual urine metals within this metal mixture and childhood SRS Z-scores at 36 months; and c) the overall effects of the metal mixture as well as the individual effects of each metal within this mixture on offspring SRS Z-scores might be heterogeneous between child sexes and/or cohort. For example, it was observed that higher levels of the overall metal mixture were associated with somewhat elevated, yet non-significant increases and decreases in SRS Z-scores in the EARLI and MARBLES cohorts, respectively. This finding may be attributed to unmeasured variations among the pregnant women in these 2 cohorts, such as those related to geographical differences. The overwhelmingly null associations between the metal mixture (Pb, Hg, Se, and Mn) as well as individual metals within the mixture and offspring SRS Z-scores are consistent with some of the existing studies. For example, in a cohort study of 371 mother-child pairs in New Hampshire, metals (arsenic [As], copper [Cu], Mn, Pb, Se, and zinc [Zn]) measured in maternal toenails at 27-week gestation (ie, second trimester) was not associated with the offspring SRS Z-scores at 36 months. 84 In another cohort study of 1237 mother-child pairs in the Republic of Seychelles, methylmercury (MeHg) measured from maternal hair at birth was not associated with offspring SRS scores. 85 However, overall, the existing evidence of the associations between metals and ASD diagnosis are more mixed. For example, Roberts et al reported that perinatal exposure to an overall metals score was associated with higher odds of ASD whereas McCanlies et al found that parental exposure to general metals was not associated with odds of ASD.17,86
Several epidemiological studies have explored the potential effect measure modification of child sex on the relationships between metals and ASD-related outcomes.17,87,88 Roberts et al found strong associations between multiple ambient metals (including Cd, Pb, Mn, and Hg) concentrations and ASD in boys when compared to girls 17 while Kalkbrenner et al did not find significant sex differences in the ambient metals-ASD associations. 87 Yet another study found positive associations between hair metals concentrations and ASD among boys in sex-stratified analyses. 88 Our research has suggested some potential heterogeneity in the results based on child sex; however, the patterns and implications are still inconclusive and require further investigation.
Our study has several strengths and innovations. First, we assembled data from 2 cohorts of families/pregnancies at enriched familial likelihood for ASD. The longitudinal sampling in these cohorts enabled us to investigate the prospective associations between prenatal exposures to metals and the postnatal outcome addressing limitations of previous publicaitons. 26 Second, we chose SRS scores, a measure of social impairment in ASD, as our primary outcome to emphasize the heterogeneity of the developmental quantitative traits about their presentation and severity of ASD.68,89 These quantitative traits share underlying etiology with ASD, 90 are correlated with known genetic and nongenetic ASD risk factors,91,92 and can increase statistical efficiency when assessing complex conditions. 93 (p2) Moreover, these traits are relatable to children who are not clinically categorized as autistic but display autistic traits. 89 This approach can not only inform ASD etiology but also developmental trajectories in general. However, it cannot be excluded that some metals may contribute to risk of the clinical syndrome of ASD or certain features of ASD, either alone or in combination with other pre-disposing factors. Third, we utilized BKMR to allow for the potential non-linear and interaction effects between different metals within a mixture. BKMR is particularly well-suited for situations where prior knowledge about interactions and nonlinearity is limited, as compared to other methods such as quantile g-computation.94,95 Additionally, this method does not require the directional homogeneity assumption, unlike other approaches such as weighted quantile sum regression.95-97
To date, the etiology of ASD remains unclear. Prenatal exposures to metals has been an emerging area for epidemiologic studies on decoding the etiology of ASD. 26 While there is sprouting evidence of the associations between ASD and prenatal metal exposures,85,98 studies examining metals as mixtures during the prenatal stage are warranted. This study seeks to address this research gap by asessing the potential mixture effects of metal exposures on childhoold ASD-related outcomes. The detection of modifiable exposures can inform prevention and policymaking, aiming to curb the rising prevalence of ASD and its costly consequences. Furthermore, this study took a stab at this difficult topic by utilizing quantitative developmental traits related to autism in children with an older sibling diagnosed with ASD. These traits not only have a shared underlying etiology with ASD, 90 but also have relevance to children who are not clinically classified as autistic but display developmental traits related to autism. 89 This approach has the potential to both inform ASD etiology and developmental trajectories in general population.
There are also some limitations of this study. First, due to the low detection proportion of Cd in our sample, we excluded it from the BKMR and linear regression models. Second, most of the children in our analytic population were non-Hispanic white and from families/pregnancies at enriched familial likelihood for ASD, limiting transportability to other race/ethnicity populations or populations without a family history of ASD. Third, there were limitations in the urine measurement of the metals in terms of timing of exposure and the sample sizes for each trimester were limited. Additionally, blood was the preferred matrix to measure Pb, but we couldn’t use the blood samples from the pregnant women due to clotting issues in the specimens collected. While urine Pb is considered to reflect the Pb diffused from plasma, it has not been accepted to completely replace blood Pb as the biomarker of internal Pb concentrations due to biological variations in the urine samples, its low correlation with blood Pb, and potential impact of glomerular filtration rate.99,100 However, urine is still a valid matrix for Pb measurement and has been commonly used in epidemiological studies. 101 Furthermore, measuring metals concentrations in urine and blood yielded comparable predictive performance in a mixture setting. 102 Still, further studies are warranted to be inclusive of populations that are underrepresented in environmental epidemiologic studies and to assess measure Pb in blood samples.
Conclusions
In this analysis of 2 elevated-probability pregnancy cohorts for ASD in the United States, all the associations between the metals within the mixture (Pb, Hg, Se, and Mn) during pregnancy and offspring SRS Z-scores at 36 months were null and/or inconsistent. Additionally, the overall effect of prenatal exposure to this mixture on offspring SRS Z-scores appeared to be modified by cohort and child sex. Despite the null findings, this study conforms to the National Institute of Environmental Health Sciences (NIEHS) strategic plan of examining human experiences through the investigation of chemical mixtures. 103 Additionally, it provides crucial insights that may facilitate the development of targeted interventions to alleviate heightened metal exposures, such as regulating chemicals as a group or mixture, rather than individually. Further studies with larger sample sizes are warranted to incorporate genetic susceptibility to metals exposure and include quantitative autistic traits other than SRS scores.
Supplemental Material
Supplemental material, sj-docx-1-ehi-10.1177_11786302231225313 for Prenatal Metal Exposures and Child Social Responsiveness Scale Scores in 2 Prospective Studies by Emma X Yu, John F Dou, Heather E Volk, Kelly M Bakulski, Kelly Benke, Irva Hertz-Picciotto, Rebecca J Schmidt, Craig J Newschaffer, Jason I Feinberg, Jason Daniels, Margaret Daniele Fallin, Christine Ladd-Acosta and Ghassan B Hamra in Environmental Health Insights
Acknowledgments
We appreciate the three reviewers for reviewing our manuscript. We thank all the participants and staff from the EARLI and MARBLES study. We are aware of the recent concern of using the language “risk” in the field of autism research and are moving away from this term. However, these two cohorts were named 10 years ago before the discussion started, so we kept the cohort names.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the National Institutes of Health (R01 ES025531, PI: Fallin). Original EARLI data collection was funded by the National Institutes of Health (R01 ES016443) and Autism Speaks (003953) and is now maintained by the Early Cohort Maintenance award (R24 ES030893, R01 ES016443). The MARBLES study was funded by the National Institutes of Health (R01 ES020392, R01 ES028089, R/U24 ES028533, and P01 ES011269) and the United States Environmental Protection Agency Science to Achieve Results (STAR) program (#RD-83329201). Research reported in this manuscript was also supported by funding provided by the Department of Epidemiology at the Johns Hopkins Bloomberg School of Public Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the university.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RJS has received funding to support the MARBLES Study from the Simons Foundation. RJS consults for the Beasley Law Firm and the Linus Technology, Inc. RJS has received travel support to present at the 35th Annual Meeting of the Organization of Teratology Information Specialists (OTIS). RJS has received compensation to serve on NIH Reviews.
Author Contributions: Conceived and designed the experiments: EXY, GBH, HEV, MDF. Performed the experiments: EXY. Analyzed the data: EXY. Assisted in data analysis: JFD, HEV, MDF, KMB, KB, RJS, IHP, JIF, GBH, CL-A. Wrote the paper: EXY. All co-authors have read and approved the final manuscript.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders–autism and developmental disabilities monitoring network, 14 sites, United States, 2002. MMWR Surveill Summ. 2007;56:12-28. [PubMed] [Google Scholar]
- 2. Maenner M. Prevalence and characteristics of autism spectrum disorder among children aged 8 years — autism and developmental disabilities monitoring network, 11 sites, United States, 2020. MMWR Surveill Summ. 2023;72:1. doi: 10.15585/mmwr.ss7202a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soke GN, Maenner MJ, Christensen D, Kurzius-Spencer M, Schieve LA. Prevalence of co-occurring medical and behavioral conditions/symptoms among 4- and 8-year-old children with autism spectrum disorder in selected areas of the United States in 2010. J Autism Dev Disord. 2018;48:2663-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism. 2015;19:814-823. [DOI] [PubMed] [Google Scholar]
- 5. Buescher AV, Cidav Z, Knapp M, Mandell DS. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatr. 2014;168:721-728. [DOI] [PubMed] [Google Scholar]
- 6. Asperger H. Die “Autistischen psychopathen” im kindesalter. Arch Für Psychiatr Nervenkrankh. 1944;117:76-136. [Google Scholar]
- 7. Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943;2:217-250. [PubMed] [Google Scholar]
- 8. Constantino JN, Frazier TW. Commentary: the observed association between autistic severity measured by the Social Responsiveness Scale (SRS) and general psychopathology – a response to Hus et al.(). J Child Psychol Psychiatry. 2013;54:695-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60:524-530. [DOI] [PubMed] [Google Scholar]
- 10. Lyall K. What are quantitative traits and how can they be used in autism research? Autism Res. 2023;16:1289-1298. Published online 2023. doi: 10.1002/aur.2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Constantino JN, Davis SA, Todd RD, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33:427-433. [DOI] [PubMed] [Google Scholar]
- 12. Tick B, Bolton P, Happé F, Rutter M, Rijsdijk F. Heritability of autism spectrum disorders: a meta-analysis of twin studies. J Child Psychol Psychiatry. 2016;57:585-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karimi P, Kamali E, Mousavi S, Karahmadi M. Environmental factors influencing the risk of autism. J Res Med Sci Off J Isfahan Univ Med Sci. 2017;22:27-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Curtis JT, Hood AN, Chen Y, Cobb GP, Wallace DR. Chronic metals ingestion by prairie voles produces sex-specific deficits in social behavior: an animal model of autism. Behav Brain Res. 2010;213:42-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang Z, Zhao Y, Li Q, et al. Developmental exposure to mercury chloride impairs social behavior in male offspring dependent on genetic background and maternal autoimmune environment. Toxicol Appl Pharmacol. 2019;370:1-13. [DOI] [PubMed] [Google Scholar]
- 16. Windham GC, Zhang L, Gunier R, Croen LA, Grether JK. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco Bay area. Environ Health Perspect. 2006;114:1438-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roberts AL, Lyall K, Hart JE, et al. Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants. Environ Health Perspect. 2013;121:978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Talbott EO, Marshall LP, Rager JR, et al. Air toxics and the risk of autism spectrum disorder: the results of a population based case-control study in southwestern Pennsylvania. Environ Health. 2015;14:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. von Ehrenstein OS, Aralis H, Cockburn M, Ritz B. In utero exposure to toxic air pollutants and risk of childhood autism. Epidemiology. 2014;25:851-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Skogheim TS, Weyde KVF, Engel SM, et al. Metal and essential element concentrations during pregnancy and associations with autism spectrum disorder and attention-deficit/hyperactivity disorder in children. Environ Int. 2021;152:106468. [DOI] [PubMed] [Google Scholar]
- 21. Yoshimasu K, Kiyohara C, Takemura S, Nakai K. A meta-analysis of the evidence on the impact of prenatal and early infancy exposures to mercury on autism and attention deficit/hyperactivity disorder in the childhood. Neurotoxicol. 2014;44:121-131. [DOI] [PubMed] [Google Scholar]
- 22. Adams JB, Baral M, Geis E, et al. The severity of autism is associated with toxic metal body burden and red Blood Cell glutathione Levels. J Toxicol. 2009;2009:532640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lewandowski TA, Bartell SM, Yager JW, Levin L. An evaluation of surrogate chemical exposure measures and autism prevalence in Texas. J Toxicol Environ Health A. 2009;72:1592-1603. [DOI] [PubMed] [Google Scholar]
- 24. Yau VM, Green PG, Alaimo CP, et al. Prenatal and neonatal peripheral blood mercury levels and autism spectrum disorders. Environ Res. 2014;133:294-303. [DOI] [PubMed] [Google Scholar]
- 25. Kern JK, Geier DA, Sykes LK, Haley BE, Geier MR. The relationship between mercury and autism: A comprehensive review and discussion. J Trace Elem -med. 2016;37:8-24. [DOI] [PubMed] [Google Scholar]
- 26. Campbell KA, Hickman R, Fallin MD, Bakulski KM. Prenatal exposure to metals and autism spectrum disorder: current status and future directions. Curr Opin Toxicol. 2021;26:39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Konikowska K, Mandecka A. Trace elements in human nutrition. Recent Advances in Trace Elements. 2018;20:339-372. [Google Scholar]
- 28. Mudipalli A, Zelikoff JT. Essential and Non-essential Metals. Springer; 2017. [Google Scholar]
- 29. Ishaque AB, Johnson L, Gerald T, et al. Assessment of individual and combined toxicities of four non-essential metals (As, Cd, Hg and Pb) in the microtox assay. Int J Environ Res Public Health. 2006;3:118-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ngu YJ, Skalny AV, Tinkov AA, et al. Association between essential and non-essential metals, body composition, and metabolic syndrome in adults. Biol Trace Elem Res. 2022;200:4903-4915. [DOI] [PubMed] [Google Scholar]
- 31. Shim YK, Lewin MD, Ruiz P, Eichner JE, Mumtaz MM. Prevalence and associated demographic characteristics of exposure to multiple metals and their species in human populations: the United States NHANES, 2007-2012. J Toxicol Environ Health A. 2017;80:502-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Woodruff TJ, Zota AR, Schwartz JM. Environmental Chemicals in pregnant women in the United States: NHANES 2003-2004. Environ Health Perspect. 2011;119:878-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kalkbrenner AE, Schmidt RJ, Penlesky AC. Environmental chemical exposures and autism spectrum disorders: a review of the epidemiological evidence. Curr Probl Pediatr Adolesc Health Care. 2014;44:277-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gundert-Remy U, Stahlmann R. The blood-brain barrier in toxicology; 2010. [Google Scholar]
- 35. Aschner M, Aschner JL. Mercury neurotoxicity: mechanisms of blood-brain barrier transport. Neurosci Biobehav Rev. 1990;14:169-176. [DOI] [PubMed] [Google Scholar]
- 36. Aschner M, Gannon M. Manganese (Mn) transport across the rat blood-brain barrier: saturable and transferrin-dependent transport mechanisms. Brain Res Bull. 1994;33:345-349. [DOI] [PubMed] [Google Scholar]
- 37. Wang B, Du Y. Cadmium and its neurotoxic effects. Oxid Med Cell Longev. 2013;2013:898034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Solovyev N, Drobyshev E, Blume B, Michalke B. Selenium at the neural barriers: a review. Front Neurosci. 2021;15:630016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anyanwu BO, Ezejiofor AN, Igweze ZN, Orisakwe OE. Heavy metal mixture exposure and effects in developing nations: an update. Toxics. 2018;6:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fewtrell LJ, Prüss-Ustün A, Landrigan P, Ayuso-Mateos JL. Estimating the global burden of disease of mild mental retardation and cardiovascular diseases from environmental lead exposure. Environ Res. 2004;94:120-133. [DOI] [PubMed] [Google Scholar]
- 41. Grosse SD, Matte TD, Schwartz J, Jackson RJ. Economic gains resulting from the reduction in children’s exposure to lead in the United States. Environ Health Perspect. 2002;110:563-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grandjean P, Landrigan P. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167-2178. [DOI] [PubMed] [Google Scholar]
- 43. Xie J, Wu S, Szadowski H, et al. Developmental Pb exposure increases AD risk via altered intracellular Ca2+ homeostasis in hiPSC-derived cortical neurons. J Biol Chem. 2023;299:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang T, Guan RL, Liu MC, et al. Lead exposure impairs hippocampus related learning and memory by altering synaptic plasticity and morphology during juvenile period. Mol Neurobiol. 2016;53:3740-3752. [DOI] [PubMed] [Google Scholar]
- 45. Karasek K. Environmental disaster in Japan. Hist Perspect St -l Univ -gr J Hist Ser II. 2013;18:10. [Google Scholar]
- 46. Amorós R, Murcia M, González L, et al. Maternal selenium status and neuropsychological development in Spanish preschool children. Environ Res. 2018;166:215-222. [DOI] [PubMed] [Google Scholar]
- 47. Castriotta L, Rosolen V, Biggeri A, et al. The role of mercury, selenium and the Se-Hg antagonism on cognitive neurodevelopment: A 40-month follow-up of the Italian mother-child PHIME cohort. Int J Hyg Environ Health. 2020;230:113604. [DOI] [PubMed] [Google Scholar]
- 48. Liu SH, Bobb JF, Lee KH, et al. Lagged kernel machine regression for identifying time windows of susceptibility to exposures of complex mixtures. Biostatistics. 2018;19:325-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Whanger PD. Selenium and the brain: a review. Nutr Neurosci. 2001;4:81-97. [DOI] [PubMed] [Google Scholar]
- 50. Yang X, Yu X, Fu H, Li L, Ren T. Different levels of prenatal zinc and selenium had different effects on neonatal neurobehavioral development. Neurotoxicol. 2013;37:35-39. [DOI] [PubMed] [Google Scholar]
- 51. Amorós R, Murcia M, Ballester F, et al. Selenium status during pregnancy: influential factors and effects on neuropsychological development among Spanish infants. Sci Total Environ. 2018;610-611:741-749. [DOI] [PubMed] [Google Scholar]
- 52. Chung SE, Cheong HK, Ha EH, et al. Maternal Blood manganese and early neurodevelopment: the mothers and children’s environmental health (MOCEH) study. Environ Health Perspect. 2015;123:717-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stokes AH, Lewis DY, Lash LH, et al. Dopamine toxicity in neuroblastoma cells: role of glutathione depletion by L-BSO and apoptosis. Brain Res. 2000;858:1-8. [DOI] [PubMed] [Google Scholar]
- 54. Desole MS, Esposito G, Migheli R, et al. Glutathione deficiency potentiates manganese toxicity in rat striatum and brainstem and in PC12 cells. Pharmacol Res. 1997;36:285-292. [DOI] [PubMed] [Google Scholar]
- 55. Erikson KM, Dobson AW, Dorman DC, Aschner M. Manganese exposure and induced oxidative stress in the rat brain. Sci Total Environ. 2004;334-335:409-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Miele M, Serra PA, Esposito G, et al. Glutamate and catabolites of high-energy phosphates in the striatum and brainstem of young and aged rats subchronically exposed to manganese. Aging Clin Exp Res. 2000;12:393-397. [DOI] [PubMed] [Google Scholar]
- 57. Montes S, Alcaraz-Zubeldia M, Muriel P, Ríos C. Striatal manganese accumulation induces changes in dopamine metabolism in the cirrhotic rat. Brain Res. 2001;891:123-129. [DOI] [PubMed] [Google Scholar]
- 58. Dickerson AS, Rotem RS, Christian MA, Nguyen VT, Specht AJ. Potential Sex differences relative to autism spectrum disorder and metals. Curr Environ Health Rep. 2017;4:405-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Messinger DS, Young GS, Webb SJ, et al. Early sex differences are not autism-specific: a baby siblings research consortium (BSRC) study. Mol Autism. 2015;6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gillberg C, Cederlund M, Lamberg K, Zeijlon L. Brief report: “The Autism Epidemic”. The registered prevalence of autism in a Swedish urban area. J Autism Dev Disord. 2006;36:429-435. [DOI] [PubMed] [Google Scholar]
- 61. Wu X, Cobbina SJ, Mao G, et al. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res. 2016;23:8244-8259. [DOI] [PubMed] [Google Scholar]
- 62. Bobb JF, Valeri L, Claus Henn B, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16:493-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Aung MT, M. Bakulski K, Feinberg JI, et al. Maternal blood metal concentrations and whole blood DNA methylation during pregnancy in the early Autism Risk Longitudinal Investigation (EARLI). Epigenetics. 2022;17:253-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hertz-Picciotto I, Schmidt RJ, Walker CK, et al. A prospective study of environmental exposures and early biomarkers in autism spectrum disorder: design, protocols, and preliminary data from the MARBLES study. Environ Health Perspect. 2018;126:117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dou JF, Middleton LYM, Zhu Y, et al. Prenatal vitamin intake in first month of pregnancy and DNA methylation in cord blood and placenta in two prospective cohorts. Epigenet Chromatin. 2022;15:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Newschaffer CJ, Croen LA, Fallin MD, et al. Infant siblings and the investigation of autism risk factors. J Neurodev Disord. 2012;4:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Constantino JN, Gruber CP. Social Responsiveness Scale: SRS-2. Western Psychological Services; 2012. [Google Scholar]
- 68. Bruni TP. Test Review: Social Responsiveness Scale–second edition (SRS-2). J Psychoeduc Assess. 2014;32:365-369. [Google Scholar]
- 69. Lyall K, Song L, Botteron K, et al. The association between parental age and autism-related outcomes in children at high familial risk for autism. Autism Res. 2020;13:998-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bölte S, Poustka F, Constantino JN. Assessing autistic traits: cross-cultural validation of the social responsiveness scale (SRS). Autism Res. 2008;1:354-363. [DOI] [PubMed] [Google Scholar]
- 71. ICPMS UI. Laboratory Procedure Manual; Published online 2007. [Google Scholar]
- 72. Schisterman EF, Vexler A, Whitcomb BW, Liu A. The limitations due to exposure detection limits for regression models. Am J Epidemiol. 2006;163:374-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lubin JH, Colt JS, Camann D, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112:1691-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Meeker JD, Hu H, Cantonwine DE, et al. Urinary phthalate metabolites in relation to preterm birth in Mexico City. Environ Health Perspect. 2009;117:1587-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993;54:615-627. [DOI] [PubMed] [Google Scholar]
- 76. Kuiper JR, O’Brien KM, Welch BM, et al. Combining urinary biomarker data from studies with different measures of urinary dilution. Epidemiology. 2022;33:533-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bobb JF, Claus Henn B, Valeri L, Coull BA. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health. 2018;17:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bobb JF. bkmr: Bayesian Kernel Machine Regression. R package version 0.2.0; Published online 2017. [Google Scholar]
- 79. Keil A. Introduction to bkmr and bkmrhat. Published 2021. Accessed April 12, 2023. https://cran.r-project.org/web/packages/bkmrhat/vignettes/bkmrhat-vignette.html
- 80. Keil A. bkmrhat: Parallel Chain Tools for Bayesian Kernel Machine Regression. R package version 1.1.1. Published online 2021. [Google Scholar]
- 81. Signes-Pastor AJ, Desai G, García-Villarino M, Karagas MR, Kordas K. Exposure to a mixture of metals and growth indicators in 6–11-year-old children from the 2013–2016 NHANES. Expo Health. 2021;13:173-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hamra G, MacLehose R, Richardson D. Markov chain Monte Carlo: an introduction for epidemiologists. Int J Epidemiol. 2013;42:627-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hamra GB, Maclehose RF, Croen L, Kauffman EM, Newschaffer C. Bayesian weighted sums: a flexible approach to estimate summed mixture effects. Int J Environ Res Public Health. 2021;18:1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Doherty BT, Romano ME, Gui J, et al. Periconceptional and prenatal exposure to metal mixtures in relation to behavioral development at 3 years of age. Env Epidemiol. 2020;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Strain JJ, Love TM, Yeates AJ, et al. Associations of prenatal methylmercury exposure and maternal polyunsaturated fatty acid status with neurodevelopmental outcomes at 7 years of age: results from the Seychelles Child Development Study Nutrition Cohort 2. Am J Clin Nutr. 2021;113:304-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. McCanlies EC, Ma CC, Gu JK, et al. The CHARGE study: an assessment of parental occupational exposures and autism spectrum disorder. Occup Environ Med. 2019;76:644-651. [DOI] [PubMed] [Google Scholar]
- 87. Kalkbrenner AE, Daniels JL, Chen JC, et al. Perinatal exposure to hazardous air pollutants and autism spectrum disorders at age 8. Epidemiology. 2010;21:631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. De Palma G, Catalani S, Franco A, Brighenti M, Apostoli P. Lack of correlation between metallic elements analyzed in hair by ICP-MS and Autism. J Autism Dev Disord. 2012;42:342-353. [DOI] [PubMed] [Google Scholar]
- 89. Landry O, Chouinard PA. Why we should study the broader autism phenotype in typically developing populations. J Cogn Dev. 2016;17:584-595. [Google Scholar]
- 90. Bralten J, van Hulzen KJ, Martens MB, et al. Autism spectrum disorders and autistic traits share genetics and biology. Mol Psychiatry. 2018;23:1205-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rosenfeld JA, Ballif BC, Torchia BS, et al. Copy number variations associated with autism spectrum disorders contribute to a spectrum of neurodevelopmental disorders. Genet Med. 2010;12:694-702. [DOI] [PubMed] [Google Scholar]
- 92. Taylor MJ, Rosenqvist MA, Larsson H, et al. Etiology of autism spectrum disorders and autistic traits over time. JAMA Psychiatr. 2020;77:936-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Comuzzie AG, Hixson JE, Almasy L, et al. A major quantitative trait locus determining serum leptin levels and fat mass is located on human chromosome 2. Nat Genet. 1997;15:273-276. [DOI] [PubMed] [Google Scholar]
- 94. Yim G, Minatoya M, Kioumourtzoglou MA, et al. The associations of prenatal exposure to dioxins and polychlorinated biphenyls with neurodevelopment at 6 months of age: multi-pollutant approaches. Environ Res. 2022;209:112757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Keil AP, Buckley JP, O’Brien KM, et al. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Env Heal Perspect. 2020;128:47004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat. 2015;20:100-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Keil AP, Buckley JP, O’Brien KM, et al. Response to “comment on ‘a quantile-based g-computation approach to addressing the effects of exposure mixtures. Env Heal Perspect. 2021;129:38002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fruh V, Rifas-Shiman SL, Amarasiriwardena C, et al. Prenatal lead exposure and childhood executive function and behavioral difficulties in project viva. Neurotoxicol. 2019;75:105-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Barbosa F, Tanus-Santos JE, Gerlach RF, Parsons PJ. A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ Health Perspect. 2005;113:1669-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Shimbo S, Zhang ZW, Moon CS, et al. Correlation between urine and blood concentrations, and dietary intake of cadmium and lead among women in the general population of Japan. Int Arch Occup Environ Health. 2000;73:163-170. [DOI] [PubMed] [Google Scholar]
- 101. Sallsten G, Ellingsen DG, Berlinger B, Weinbruch S, Barregard L. Variability of lead in urine and blood in healthy individuals. Environ Res. 2022;212:113412. [DOI] [PubMed] [Google Scholar]
- 102. Ashrap P, Watkins DJ, Mukherjee B, et al. Performance of urine, blood, and integrated metal biomarkers in relation to birth outcomes in a mixture setting. Environ Res. 2021;200:111435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. National Institute of Environmental Health Sciences (NIEHS). 2018-2023 Strategic Plan: Advancing Environmental Health Science, Improving Health 2.0. Dep Health Hum Serv Ed Durh NC. Published online 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ehi-10.1177_11786302231225313 for Prenatal Metal Exposures and Child Social Responsiveness Scale Scores in 2 Prospective Studies by Emma X Yu, John F Dou, Heather E Volk, Kelly M Bakulski, Kelly Benke, Irva Hertz-Picciotto, Rebecca J Schmidt, Craig J Newschaffer, Jason I Feinberg, Jason Daniels, Margaret Daniele Fallin, Christine Ladd-Acosta and Ghassan B Hamra in Environmental Health Insights
Data Availability Statement
Data requests should be addressed to the Principal Investigators of the 2 cohorts (EARLI: MDF; MARBLES: RJS). After the paper is accepted for publication, the code will be made publicly available at https://github.com/emmayu001/BKMR-Metals-SRS.