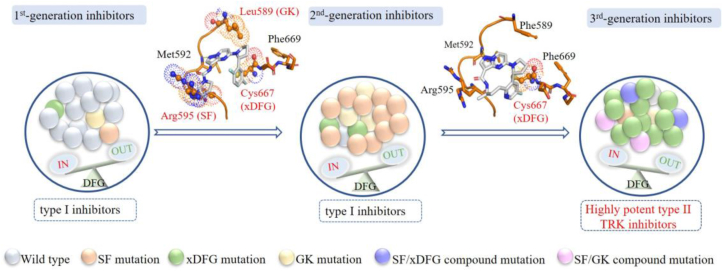
Figure 6.
Proposed therapeutical pipeline for NTRK fusion cancers. Upon diagnosed, tumors mainly harbor wild-type NTRK fusion cells. These wide-type kinases adopt a DFG-in active conformation and type I based 1st-generation TRK inhibitors larotrectinib or entrectinib are approved for use. Subsequently developed point mutations resistances and SF alterations dominate. The kinases remain in DFG-in active conformation and type I based 2nd-generation inhibitors are applied. At progression, the emerged xDFG mutations and some compound mutations cause steric clashes, impeding the binding of type I based 2nd-generation inhibitors, and induce the kinase preferring a DFG-out inactive conformation, favoring the binding of type II inhibitors. Each colored ball represents a distinct clone. Steric clashes are highlighted in spheres.