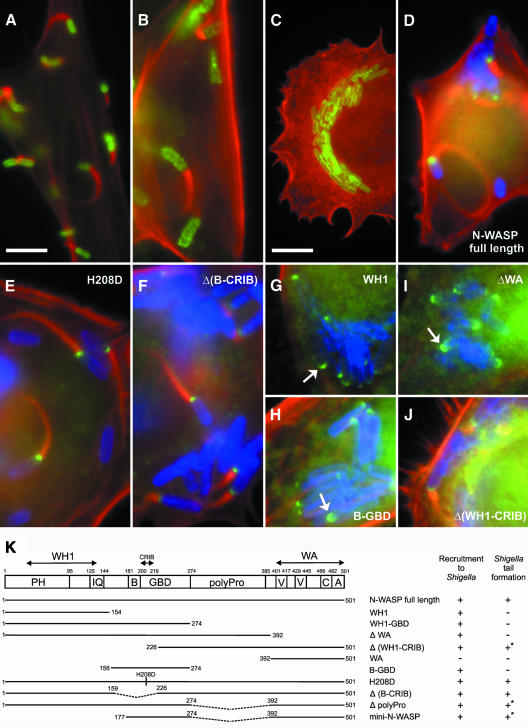
Fig. 3. N-WASP is essential for Shigella-induced actin tail formation. Precursor (B) and N-WASP-defective fibroblasts (A and C–J) were infected with L. monocytogenes (A) or S. flexneri (B–J). (A–C) show non-transfected cells, while (D–J) show cells expressing GFP-tagged N-WASP constructs as indicated. In all images, filamentous actin is shown in red. Listeria (A) and Shigella (B, C) are shown in green. In (D–J), Shigella and GFP fusion proteins are labeled blue and green, respectively. Listeria form actin tails in N-WASP-defective fibroblasts (A), while Shigella-induced actin tails are abolished in these cells (C), in contrast to the precursor cell line (B). Actin tail formation by Shigella is restored upon expression of full-length N-WASP (D), H208D, Δ(B-CRIB) and Δ(WH1-CRIB) mutants (E, F and J, respectively), but not after expression of N-WASP-WH1 (G), -B-GBD (H) or N-WASP-ΔWA (I), although the three latter constructs are recruited to the Shigella surface (indicated by arrows). Bar in (A) (5 µm) is valid for (A–J) except (C) (10 µm). (K) shows the domain structure of N-WASP and an overview of the GFP-tagged constructs used in this study. Recruitment to Shigella and reconstitution of actin tail formation are given. +* marks low efficiency and/or shorter tails; PH, pleckstrin-homology domain; IQ, calmodulin-binding motif; B, basic domain; GBD, GTPase binding domain; polyPro, polyproline region; V, verprolin homology domain; C, cofilin homology domain and A, acidic domain.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.