Figure 2:
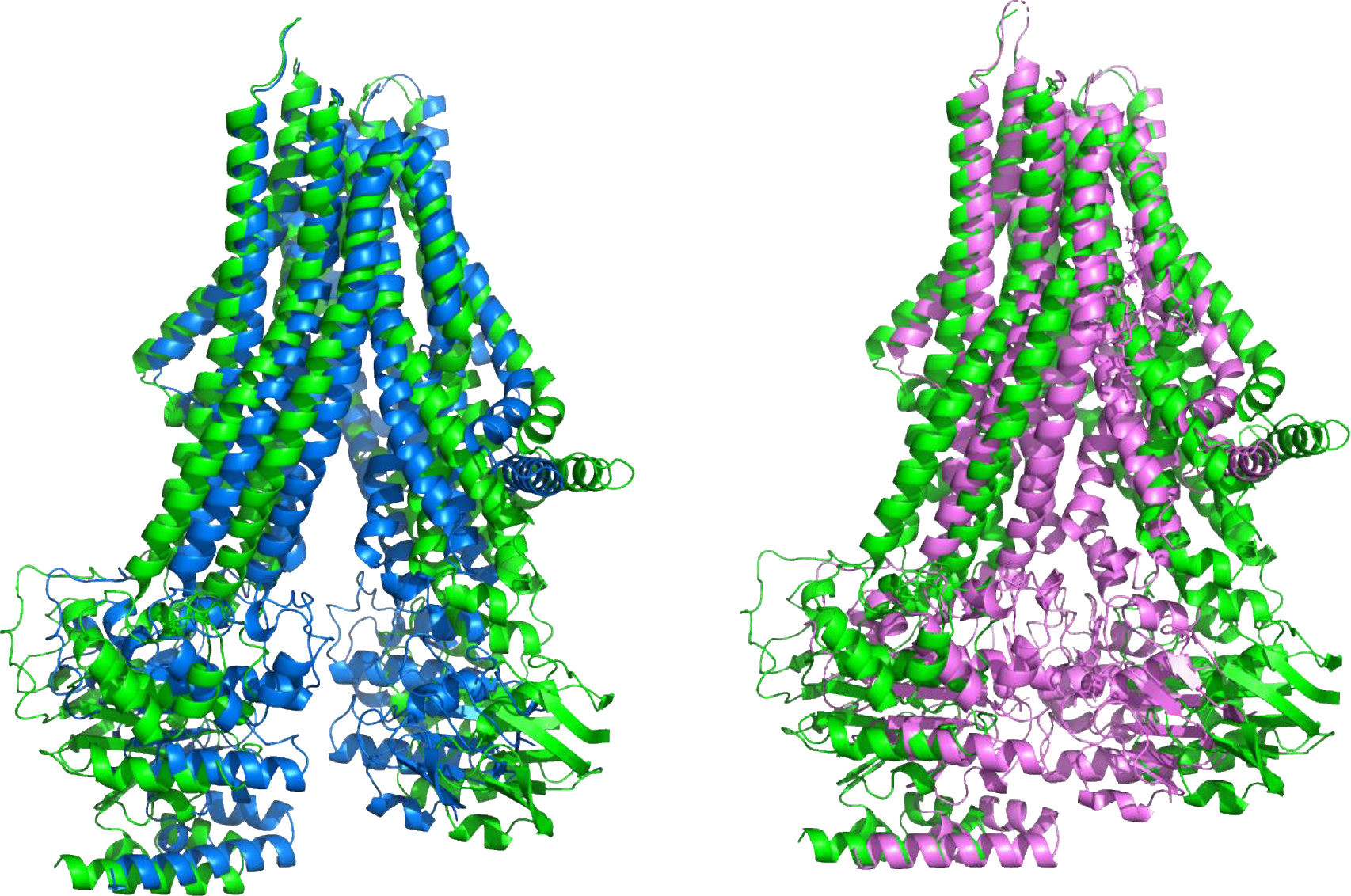
Superposition of the experimental apo open structure (T1158, green) of the multidrug resistance Type IV ABC transporter and the structure with a bound DHEAS transported (T1158v3, Blue, left) and with bound ATP-Mg (T1158v4, Magenta, right). The structures differ mainly in the inter-domain angle of the transmembrane domains: the green apo structure is the most open, binding of transported ligands results in a partial closure (blue, left), and ATP-Mg (magenta, right) binding produces a closed structure with the ATP binding domains (bottom of the molecule in this view) interacting. Several groups successfully reproduced this three-member conformational ensemble.
