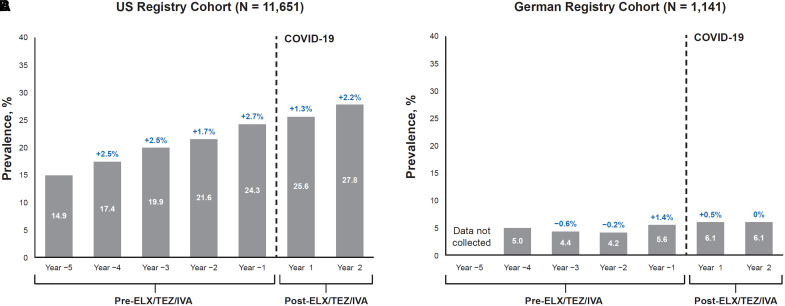
Figure 1.
Prevalence of depression reported in the elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) postauthorization safety study (PASS) longitudinal cohort before and after initiation of ELX/TEZ/IVA use. (A and B) Results using data from people with cystic fibrosis (pwCF) in the U.S. Cystic Fibrosis Foundation Patient Registry (A) and the German Cystic Fibrosis Registry (Mukoviszidose eV) (B). Time periods include 5 years before ELX/TEZ/IVA initiation and 2 years (Year 1 and Year 2) after starting ELX/TEZ/IVA treatment. Change in prevalence of depression from the previous year is indicated in blue. COVID-19 = coronavirus disease.