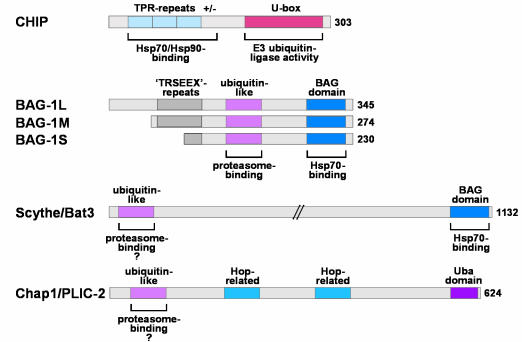
Fig. 1. Cofactors that appear to link molecular chaperones to the ubiquitin/proteasome system. CHIP possesses an N-terminal chaperone binding motif formed by three TPRs and an adjacent highly charged region. A U-box required for ubiquitin ligase activity is present at the C-terminus. The BAG-1 isoforms share a ubiquitin-like domain involved in proteasome binding and a BAG domain that mediates interaction with Hsp70. Like the BAG-1 proteins, Scythe/Bat3 possesses a ubiquitin-like domain that may be used for proteasome association and a BAG domain used for binding and regulation of Hsp70. Chap1/PLIC-2 combines a ubiquitin-like domain and a Uba domain, the latter of which is found in several proteins involved in ubiquitin conjugation. In addition, regions structurally related to the chaperone cofactor Hop are present in Chap1/PLIC-2.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.