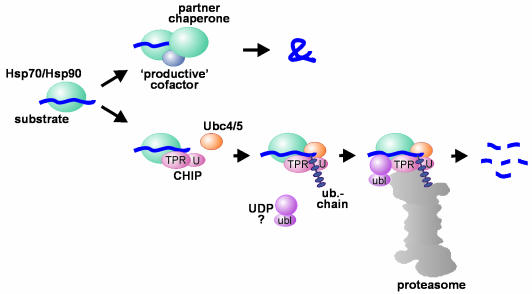
Fig. 2. Hsp70 and Hsp90 in protein folding and degradation. An initial decision to fold or degrade a Hsp70- or Hsp90-associated substrate protein may be reached through competition between a ‘productive’ cofactor such as Hop, and the ubiquitin ligase CHIP. During the folding process, Hsp70 and Hsp90 may co-operate with other chaperone proteins (termed partner chaperones). On the degradation pathway, CHIP associates with Hsp70 or Hsp90 via its TPR chaperone adaptor (TPR), and at the same time recruits E2 ubiquitin conjugating enzymes of the Ubc4/5 family to the chaperone complex. This may involve binding of the E2 to the U-box of the cofactor (U). In conjunction with E2, CHIP mediates ubiquitin attachment to the chaperone substrate and induces its targeting to the proteasome for degradation. The targeting process may be facilitated by a ubiquitin domain protein (UDP), such as BAG-1, which binds to Hsp70 and utilizes its ubiquitin-like domain (ubl) for proteasomal association.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.