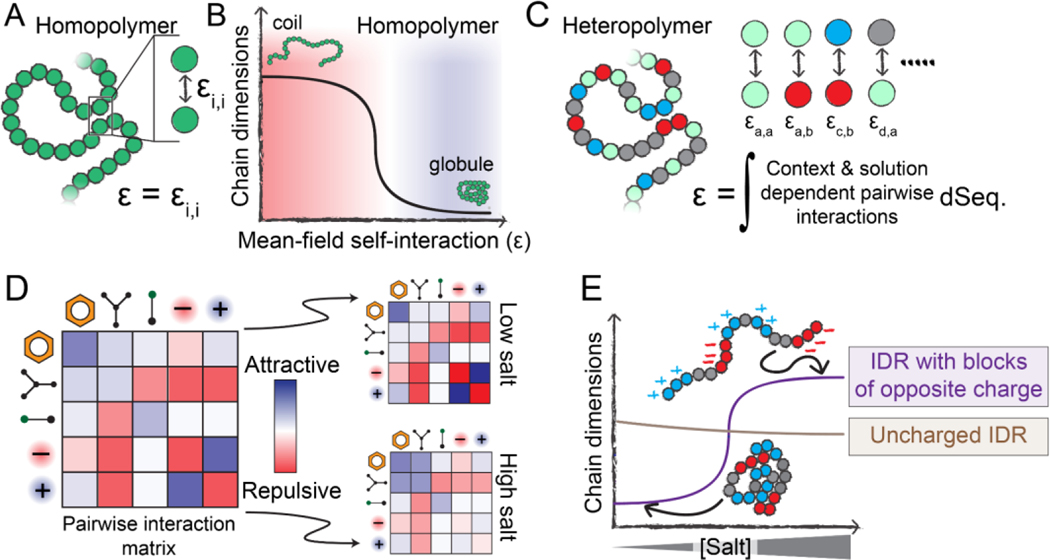
Figure 2. Physical principles that underlie sequence-specific IDR sensitivity to changes in physicochemistry.
(A) Homopolymers are defined by a single interaction strength between each polymer unit, which also defines the mean-field self-interaction energy (ε). (B) If ε is repulsive, a homopolymer behaves as an extended coil with large chain dimensions, whereas if ε is attractive, a homopolymer behaves as a compact globule. The mean-field interaction energy can be varied by changing the chemical identity of the polymer unit, but can also be varied by altering the physicochemical environment the polymer finds itself in (temperature, pH, solutes, etc.). (C) Unlike homopolymers, heteropolymers consist of many chemically distinct units. A complete description of a heteropolymer requires knowledge of how each unique inter-residue interaction behaves, and the mean-field self-interaction energy (ε) is now defined in terms of the composition-weighted and context-dependent integral over all possible interactions. (D) The various types of interactions that may occur between residues in a heteropolymer can be, to first order, described by an interaction matrix. The strengths of these interactions depend on solution conditions. (E) The response of a heteropolymer to changes in the solution environment depends on the heteropolymer’s chemistry. For example, a highly-charged IDR with blocks of oppositely-charged residues will be compact at low salt due to strong intramolecular electrostatic interactions. However, under high-salt conditions, those attractive interactions are screened, leading to an expanded ensemble driven by the substantial solvation free energies associated with charged groups. In contrast, a charge-depleted heteropolymer may be relatively salt-insensitive and is relatively compact compared to the blocky IDR in the high-salt limit.