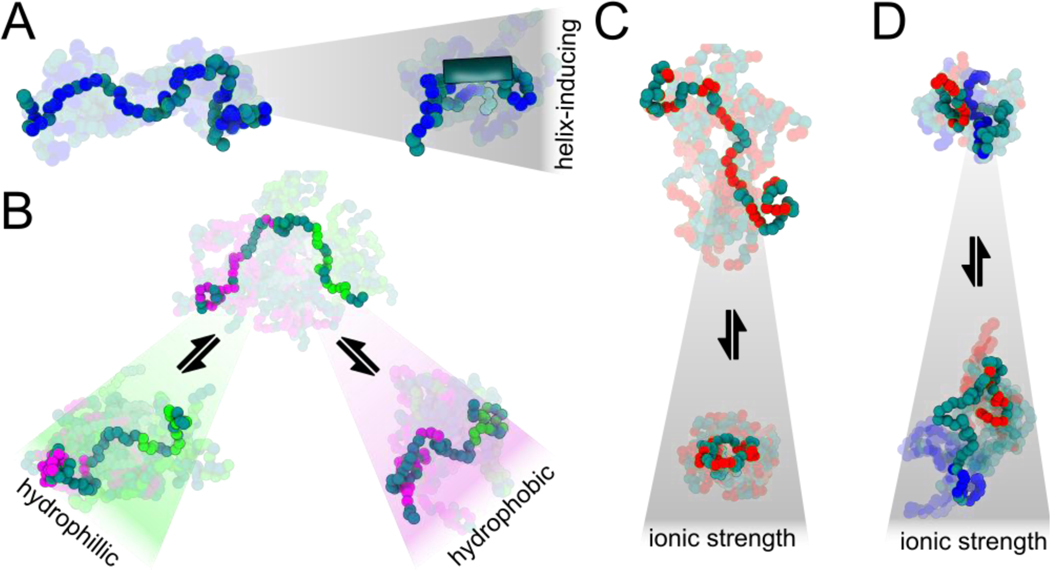
Figure 4. Examples of physicochemically-driven changes in IDR ensembles.
(A) Promotion of secondary structural elements such as residual helicity (shown by the tube on the right) can form or dissolve binding motifs, modulating binding affinities. By prepaying an entropic cost for binding, the effective concentration of binding motifs can be rapidly enhanced or suppressed without the need to alter protein copy number. (B) Amphipathic sequences with patches of hydrophobic or hydrophilic residues can compact or expand locally in different solutions, tuning accessibility of specific regions. (C) Sequences with high net charge (positive or negative) can compact when an increase in ionic strength screens out repulsive interactions. (D) Charged sequences with sequestered, opposite charges can expand at high ionic strength due to screening of attractive interactions.