Figure 1.
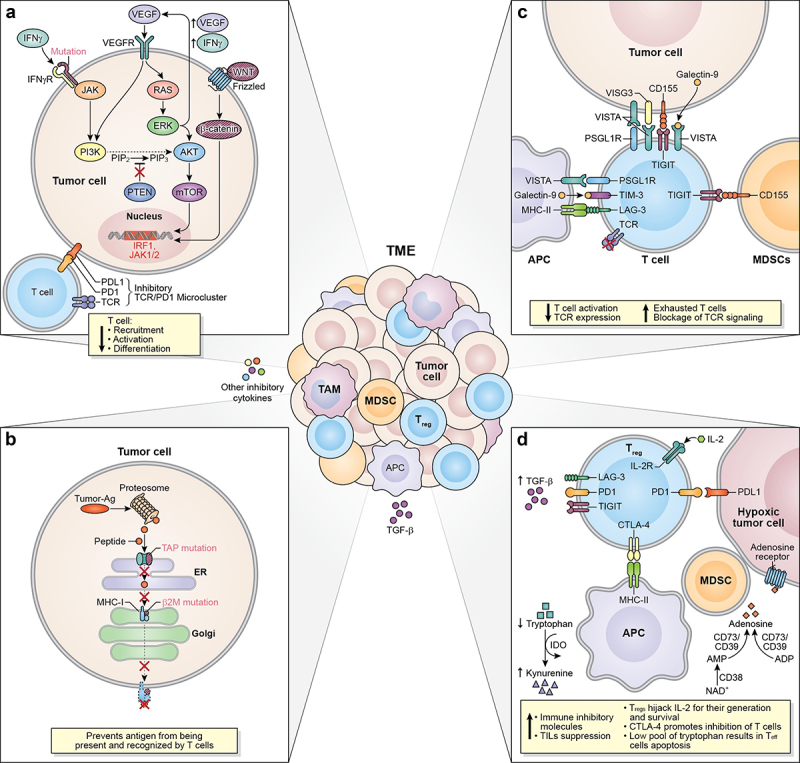
Mechanism associated to ICI resistance. The TME represents a complex interaction between tumor cells, immune cells, and inhibitory cytokines leading to a plethora of mechanisms associated to ICI resistance. (a) Defects in T cells effector function and their exclusion from the TME can occur as a result of dysregulation of MAPK/ERK cell signaling pathways in tumor cells inducing the production of VEGF and IL-8, having a net inhibitory effect on the recruitment of T cells and defects in T-cell activation and differentiation. Due to the crosstalk between the MAPK and the PI3K signaling pathways, an oncogenic mutation in MAPK pathway or a loss of PTEN expression can cause the enhancement of PI3K-AKT signaling as well. Similarly, a mutation or silencing of -catenin or tumor suppressor wnt protein, results in dysregulation of the WNT/-catenin pathway, promoting aberrant signaling of b -catenin contributing to the absence of T cell expression signature. Mutations in the IFN-g receptor and defects on the IRF1 or JAK1/2 genes can contribute to T cell desensitization and consequently promote acquired resistance to ICIs. Specifically, mutations of PD-L1, PD-L2, and JAK2 genes resulting from the amplification of the locus that contains these genes promote the formation of the PD-1/TCR inhibitory microcluster, which results in the inhibition of T cell activation. (b) Another resistance mechanism is developed after defects in the APM specifically due to mutations of B2M and TAP proteins. These mutations make the antigen unable to reach the tumor cell’s surface and be recognized and cleared out by CD8+ T cells. (c) the interaction between T cells, tumor cells, APCs, and immunosuppressive cells (MDSCs) throughout immune checkpoints such as VISTA, LAG-3, TIGIT, and TIM-3, triggers and inhibitory signal causing the exhaustion of T cells, blockage of TCR signaling, and decreasing T cell activation and TCR expression. (d) Immunosuppressive signaling also contributes to primary and/or acquired resistance in cancer. Tregs can restrain the effector function of immunocompetent cells by inducing checkpoint-mediated suppression (CTLA-4, PD-1, TIGIT, TIM-3, and LAG-3), competing for IL-2 binding, or by producing anti-inflammatory cytokines. As such, TGF-b is a pleiotropic cytokine involved in tumor evasion and immunotherapy resistance. The upregulation of the metabolic modulator IDO within the TME allows the depletion of tryptophan, resulting in the decrease of T cells. Another metabolic modulator associated with T cell function suppression and ICI resistance is adenosine. Several ectonucleotidases (CD39 and CD73) catalyze the conversion of ADP or AMP to adenosine or NAD+ to AMP (CD38) causing the upregulation of adenosine in the tumor milieu. TME: tumor microenvironment; MAPK/ERK: mitogen-activated protein kinase/extracellular signal-regulated kinase; VEGF: vascular endothelial growth factor; IL-8: interleukin-8; PTEN: phosphatase and tensin homolog deleted on chromosome 10; PI3K-AKT: phosphoinositide-3-kinase – protein kinase B/Akt; WNT/-catenin: wingless-related integration site/ b-catenin; IRF1: interferon regulatory factor 1; JAK1/2: Janus kinase 1/2; PD-L1/L2: program cell-death ligand 1/ligand 2; PD-1/TCR inhibitory microcluster: program cell-death 1/T cell receptor inhibitory microcluster; APM: antigen presenting machinery; 2 M; 2-microglobulin; TAP:transporter associated with antigen presentation; APC: antigen presenting cells; MDSCs: myeloid-derived suppressor cells; VISTA: V-domain ig suppressor of T cell activation; LAG-3: lymphocyte activation gene 3; TIGIT: T cell ImmunoGlobulin and ImmunoTyrosine inhibitory motif (ITIM) domain; TIM-3: T cell immunoglobulin and mucin-3; IDO: indoleamine 2,3-dioxygenase 1; CTLA-4:cytotoxic T lymphocyte antigen-4; TGF-: transforming growth factor beta ; ADP: adenosine triphosphate; AMP: adenosine monophosphate; NAD+: nicotinamide adenine dinucleotide.
