Figure 2.
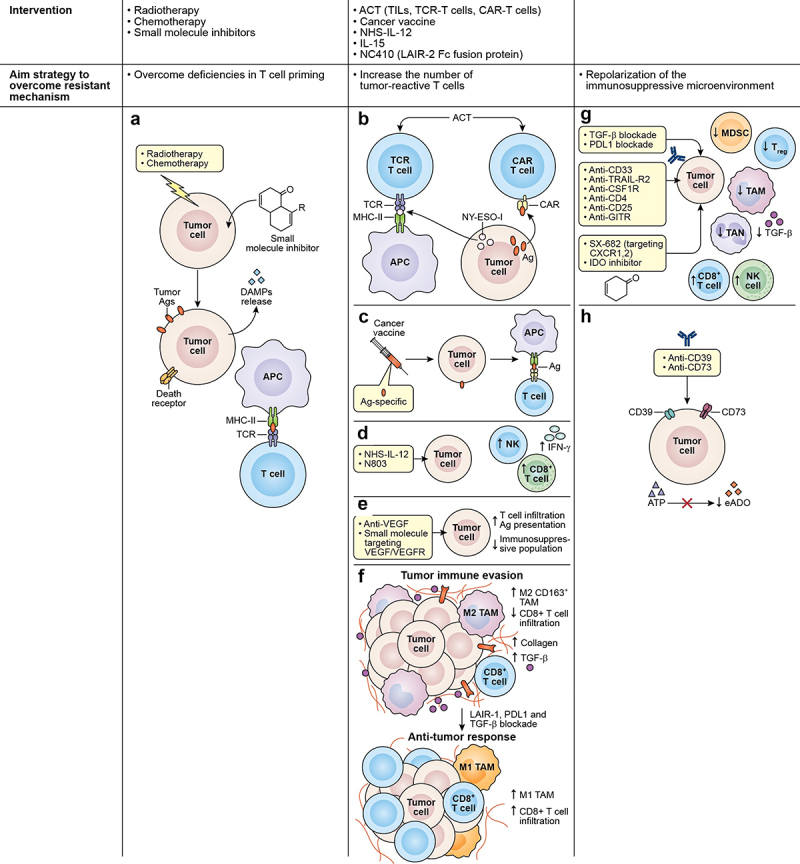
Strategies to overcome associated mechanisms contributing to immunotherapy resistance. (a) Chemotherapy, radiotherapy, and small molecule inhibitors are among the strategies used to overcome deficiencies in T-cell priming. These agents can induce ICD in tumor cells causing the release of antigens and DAMPs, allowing the recruitment and maturation of APCs and the presentation of targetable antigens to effector T cells. Other interventions are used to increase the number of tumor-reactive T cells interacting in the TME, such as (b) ACT using TCR T cell or CAR T cell. TCR T directed against specific cancer antigens (testis antigen, NY-ESO-1) can recognize the antigen through MCH molecules and CAR T cells act in an MHC-independent manner targeting cell surface antigens. (c) the use of cancer vaccines is also used to expand tumor-specific T cell populations, broaden the T cell repertoire, and promote the transport of T cells to tumor lesions. Additionally, the use of (d) immunostimulatory cytokines such as NHS-IL-12 or N803 can promote the enhancement of effector cell recruitment and boost CD8+ T cell and NK cell cytolytic functions. (e) physical barriers also prevent the infiltration of effector T cells in the TME, and tumor vasculature represents one of these barriers. Therefore, VEGF targeting using small molecule inhibitors or mAbs represents an effective strategy to disrupt angiogenic pathways that are fostering the aberrant vasculature. (f) another physical barrier is the extracellular matrix that is composed mainly of collagen. Collagen is produced by TAMs, CAFs, and tumor cells and can impair immune activity through interactions with LAIR-1. Blockade of LAIR-1 combined with PD-1, and TGF- blockade can increase M1 TAMs population, CD8+ T cell infiltration, while decreasing TGF- and collagen. Figure adapted from J Clin Invest. 2022;1328: e155148. https://doi.Org/10.1172/JCI155148. lastly, repolarization of the immunosuppressive microenvironment as an alternative strategy to overcome certain resistant mechanisms can be achieved by (g) the use of mAbs targeting CD33 and TRAIL-R2 receptors expressed in tumor cells were demonstrated to eliminate MDSCs. The use of anti-CFR-1 were demonstrated to eliminate M2 macrophages. Likewise, the depletion of tregs using anti-CD4, anti-CD25 or anti-GITR in combination with ICI have shown to improve CD8+ T cell activity. Another approach uses to decrease the immunosuppressive population that abrogates the effect of ICIs, is the use of small molecule inhibitors targeting CXCR1/2 which are receptors to chemokines essential for the recruitment of MDSCs and TANs. Growing evidence have shown that blocking TGF-b and PD-L1 simultaneously can decrease immunosuppressive population in the TME. (h) the use of inhibitors against IDO enzyme, halt the conversion of tryptophan to kynurenine allowing the increase of NK cells and CD8+ T cell and the decrease of Tregs.
ICD: immunogenic cell death; DAMPs: damage-associated molecular patterns; APC: antigen presenting cells; TME: tumor microenvironment; ACT: adoptive cell transfer; TCR T: T cell receptor-engineered T cells; CAR T cells: chimeric antigen receptor T cells; MCH: major histocompatibility complex; VEGF: vascular endothelial growth factor; TAMs: tumor-associated macrophages; LAIR-1: leukocyte-associated immunoglobulin-like receptor-1; CAFs: cancer-associated fibroblasts; TANs: tumor-associated neutrophils; PD-1: program cell-death 1; TGF-:transforming growth factor beta.
