Figure 2. Schematic of Gen 1–2 trial conduct.
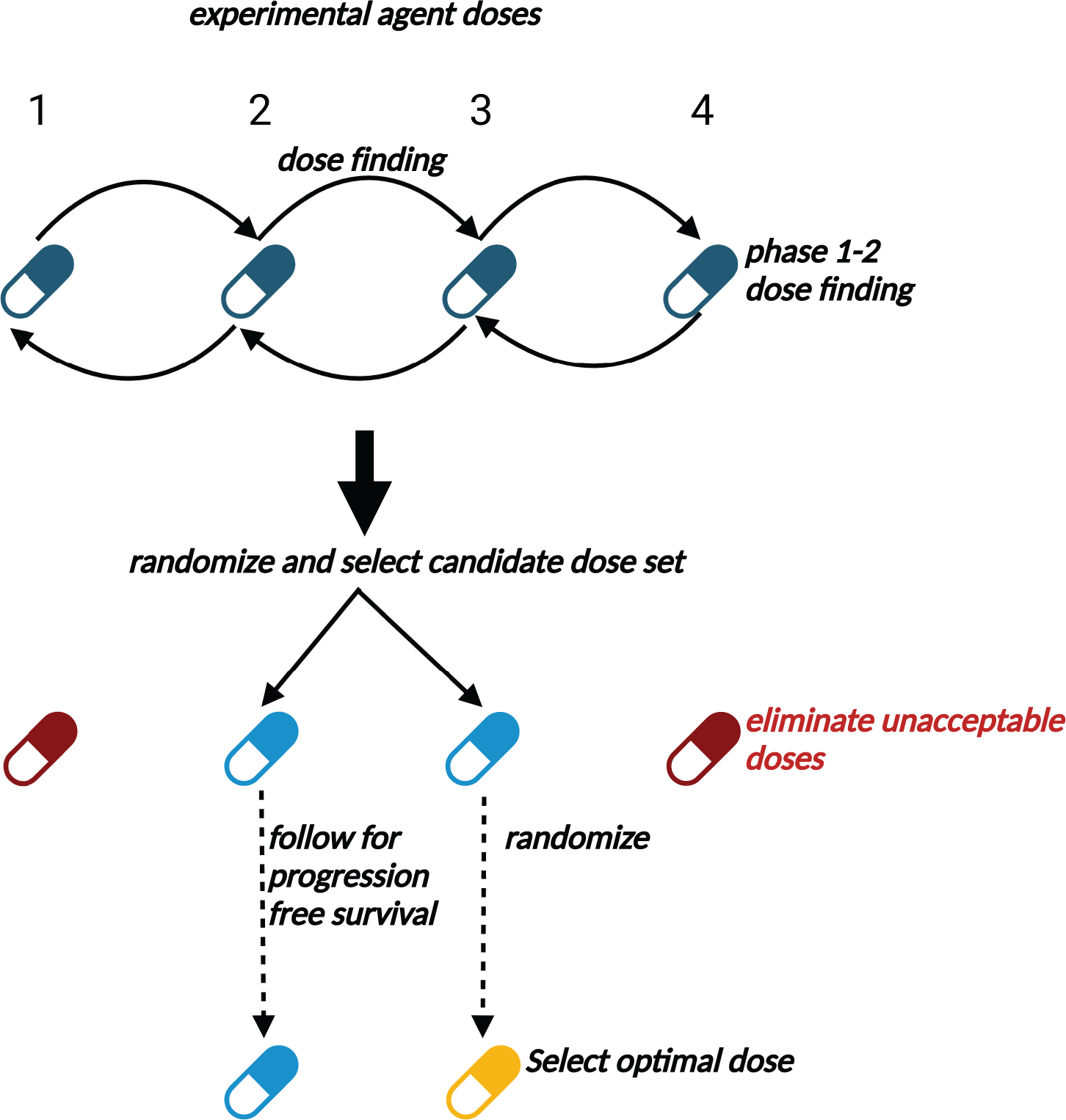
In Stage 1, doses are assigned to successive patient cohorts using typical phase 1–2 design rules to optimize early efficacy and toxicity. The set of doses found to be acceptable during Stage 1 then are randomly assigned among patients in Stage 2 to select a set of acceptable doses and eliminate unacceptable doses more accurately. Additional patients then are randomized among the remaining acceptable doses and followed over an extended time period to establish efficacy in terms of long-term outcomes such as progression-free survival. The candidate dose with the best estimated long-term outcome is ultimately chosen.
