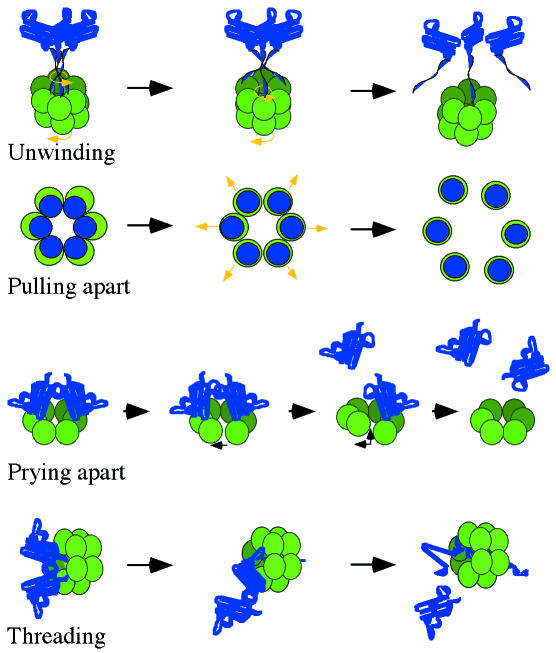
Fig. 2. Proposed mechanisms for AAA proteins. Blue represents substrate and green AAA proteins. (A) Twisting motions between D1 and D2 domains or between N-domains and D1 could lead to unwinding of helical substrate bundles, as is proposed for SNARE disassembly. (B) A pulling apart mechanism, proposed for microtubule disassembly, requires that the substrate components form stable interactions with separate AAA subunits and consequently dissociate from each other, pulled along as the AAA subunits separate. (C) Prying apart of subunits could occur upon partial ring opening, as seems to be the case for DNA clamp loaders. Nucleotide-dependent conformational changes could introduce tension in the ring, causing subunits to separate and forcing bound substrate subunits apart. (D) Threading occurs by unraveling of the protein substrate from one end, as in vectorial translocation of proteins by Clp ATPases. Movement of the polypeptide chain could occur by repeated cycles of substrate binding and release within the channel.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.