Fig. 2. TMEM106B core deposition associates with risk haplotype.
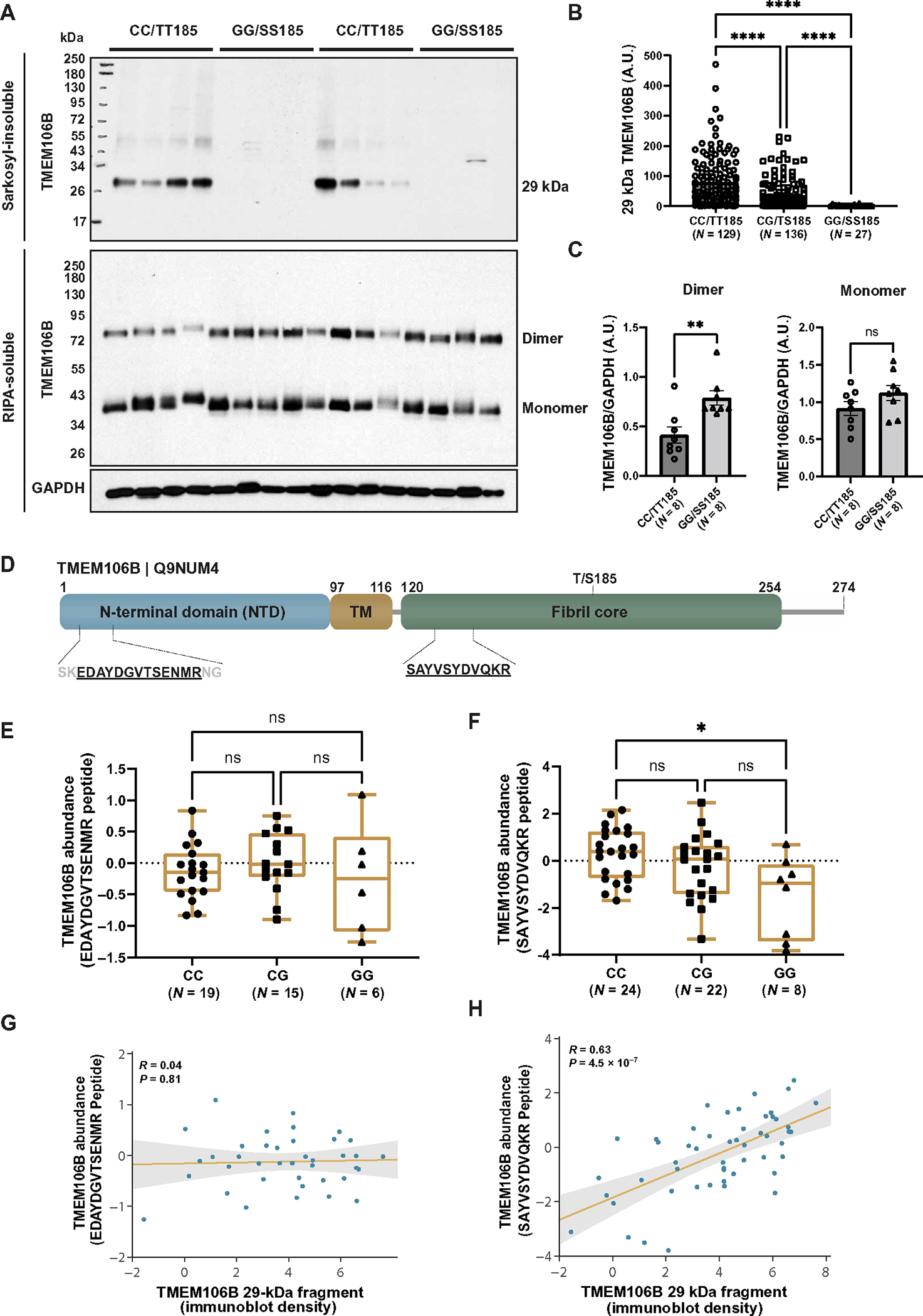
(A) The sarkosyl-insoluble fraction was extracted from the frontal cortices of patients with FTLD-TDP (n = 292 total: 129 CC, 136 CG, and 27 GG) and separated by SDS-PAGE (top). In addition, the radioimmunoprecipitation assay (RIPA)–soluble fraction from the frontal cortices of FTLD-TDP patients (n = 16 total: 8 CC and 8 GG) was separated by SDS-PAGE using cold conditions to visualize both dimeric and monomeric forms of TMEM106B (bottom). Immunoblotting was performed using TMEM106B and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (RIPA-soluble fraction only) antibodies. (B) Quantification was performed on the 29-kDa TMEM106B-positive fragment (A, top). Data presented as means ± SEM, with statistical analysis performed using a one-way ANOVA with Dunn’s multiple comparisons (****P < 0.0001). (C) Quantification was performed on full-length TMEM106B dimer (left) and monomer (right), using GAPDH to control for protein loading [immunoblots presented in (A, bottom)]. Data presented as means ± SEM, with statistical analysis performed using a Student’s t test (**P < 0.01). ns, not significant; A.U., arbitrary units. (D) Schematic diagram of the TMEM106B protein illustrating the N-terminal domain, transmembrane domain (TM), and C-terminal fibril core. Location of peptide sequences identified by mass spectrometry are depicted. (E and F) Quantification of N-terminal (E) or C-terminal (F) peptides mapped to the TMEM106B protein demonstrate abundance in the sarkosyl-insoluble fraction of FTLD-TDP cases stratified by rs3173615 genotype. Fifty-four cases were processed for mass spectrometry. The N-terminal peptide was detected in 19 CC, 15 CG, and 6 GG cases and was not detected in 14 cases. The C-terminal core domain peptide was detected in all cases (n = 24 CC, 22 CG, and 8 GG). Statistical analysis performed using the Kruskal-Wallis test with Dunn’s multiple comparisons (*P < 0.05). (G and H) Regression analysis of N-terminal (G) or C-terminal (H) peptides with 29-kDa TMEM106B fragment quantification by immunoblot.
