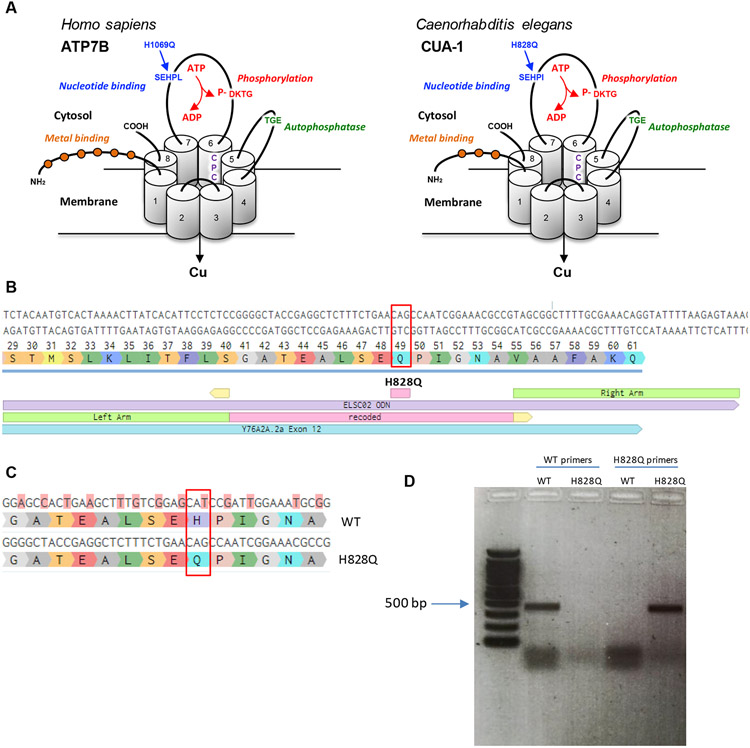
Figure 1. Generation of the H828Q substitution in CUA-1.
A. Three-dimensional representation of the human ATP7B and its C.elegans ortholog CUA-1. The proteins have eight transmembrane (TM) domains, which form a pore for transport of Cu across the membrane. The CPC motif in 6th transmembrane domain plays a key role in the metal translocation along the pore. ATP7B and CUA-1 contain also ATP-binding (blue), phosphatase (green), phosphorylation (red) domain and N-terminal tail with metal binding sites (orange balls). Arrows indicate conservative histidine in nucleotide binding domain, which is substituted in large cohort of WD patients and was targeted in cua-1(knu790[H828Q]) C.elegans strain. B. The panel shows a portion of exon 12 with coding triplets and site of H828Q substitution (red rectangle). The scheme below shows the alignament of donor DNA template (in violet), used for knockin generation, with recoded region (pink) and homology arms (green). The sights of sgRNA guided cut are shown in yellow. C. The scheme shows a portion of exon 12 with alignament of WT cua-1 with recoded region in the DNA template. The substituted nucleotides are highlighted in pink and result in silent mutations with exception of CAT (histidine) to CAG (glutamate) codon change. D. Agarose gel electrophoresis of RT-PCR products amplified from WT and cua-1(knu790[H828Q]) RNAs using either WT-specific or H828Q-specific cua-1 primers.